Label: ERYTHROMYCIN ointment
- NDC Code(s): 55154-7850-0
- Packager: Cardinal Health 107, LLC
- This is a repackaged label.
- Source NDC Code(s): 24208-910
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
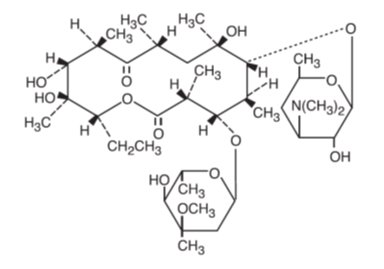
Erythromycin ophthalmic ointment, USP belongs to the macrolide group of antibiotics. The sterile ophthalmic ointment flows freely over the conjunctiva. Erythromycin base, as crystals or powder, is slightly soluble in water, moderately soluble in ether, and readily soluble in alcohol or chloroform. Erythromycin is an antibiotic produced from a strain of Streptomyces erythraeus. It is basic and readily forms a salt when combined with an acid. It has the following structural formula:
Molecular Formula: C37H67NO13
Mol. Wt. 733.94
Chemical Name: ((3R●,4S●,5S●,6R●,7R●,9R●,11R●,12R●,13S●,14R●)-4-[(2,6-dideoxy-3-C-methyl-3-0-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione)
Each gram contains: Active: erythromycin USP, 5 mg (0.5%); Inactives: mineral oil and white petrolatum.
-
CLINICAL PHARMACOLOGY
Microbiology
Erythromycin inhibits protein synthesis without affecting nucleic acid synthesis. Erythromycin is usually active against the following organisms in vitro and in clinical infections: Streptococcus pyogenes (group A β-hemolytic), Alpha-hemolytic streptococci (viridans group); Staphylococcus aureus, including penicillinase-producing strains (methicillin-resistant staphylococci are uniformly resistant to erythromycin); Streptococcus pneumoniae;Mycoplasma pneumoniae (Eaton Agent, PPLO); Haemophilus influenzae (not all strains of this organism are susceptible at the erythromycin concentrations ordinarily achieved); Treponema pallidum; Corynebacterium diphtheriae; Neisseria gonorrhoeae; Chlamydia trachomatis.
-
INDICATIONS AND USAGE
For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by organisms susceptible to erythromycin.
For prophylaxis of ophthalmia neonatorum due to N. gonorrhoeae or C. trachomatis.
The effectiveness of erythromycin in the prevention of ophthalmia caused by penicillinase-producing N. gonorrhoeae is not established.
For infants born to mothers with clinically apparent gonorrhea, intravenous or intramuscular injections of aqueous crystalline penicillin G should be given: a single dose of 50,000 units for term infants or 20,000 units for infants of low birth weight. Topical prophylaxis alone is inadequate for these infants.
- CONTRAINDICATIONS
-
PRECAUTIONS
General
The use of antimicrobial agents may be associated with the overgrowth of non-susceptible organisms including fungi; in such a case, antibiotic administration should be stopped and appropriate measures taken.
Information for Patients: Avoid contaminating the applicator tip with material from the eye, fingers, or other source.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Two year oral studies conducted in rats with erythromycin did not provide evidence of tumorigenicity. Mutagenicity studies have not been conducted.
No evidence of impaired fertility that appeared related to erythromycin was reported in animal studies.
Pregnancy: Reproduction studies have been performed in rats, mice, and rabbits using erythromycin and its various salts and esters, at doses that were several multiples of the usual human dose. No evidence of harm to the fetus that appeared related to erythromycin was reported in these studies. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, the erythromycins should be used during pregnancy only if clearly needed.
Nursing Mothers: Caution should be exercised when erythromycin is administered to a nursing woman.
Pediatric Use: See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
In the treatment of superficial ocular infections, erythromycin ophthalmic ointment approximately 1 cm in length should be applied directly to the infected eye(s) up to six times daily, depending on the severity of the infection.
For prophylaxis of neonatal gonococcal or chlamydial ophthalmia, a ribbon of ointment approximately 1 cm in length should be instilled into each lower conjunctival sac. The ointment should not be flushed from the eye following instillation. A new tube should be used for each infant.
-
HOW SUPPLIED
Erythromycin ophthalmic ointment USP, 0.5% is available in the following sizes:
Overbagged with 10 x 1 Gram Unit Dose Tin Tubes in each bag, NDC 55154-7850-0
Storage: Store between 15°C to 25°C (59°F to 77°F).
Keep out of reach of children.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
© 2023 Bausch & Lomb Incorporated or its affiliates
Distributed By:
Cardinal Health
Dublin, OH 43017
L28625630424
Revised: May 2023
9795000 (Folded)
9795100 (Flat)
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
ERYTHROMYCIN
erythromycin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55154-7850(NDC:24208-910) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERYTHROMYCIN (UNII: 63937KV33D) (ERYTHROMYCIN - UNII:63937KV33D) ERYTHROMYCIN 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55154-7850-0 10 in 1 BAG 07/29/1994 1 1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064067 07/29/1994 Labeler - Cardinal Health 107, LLC (118546603)