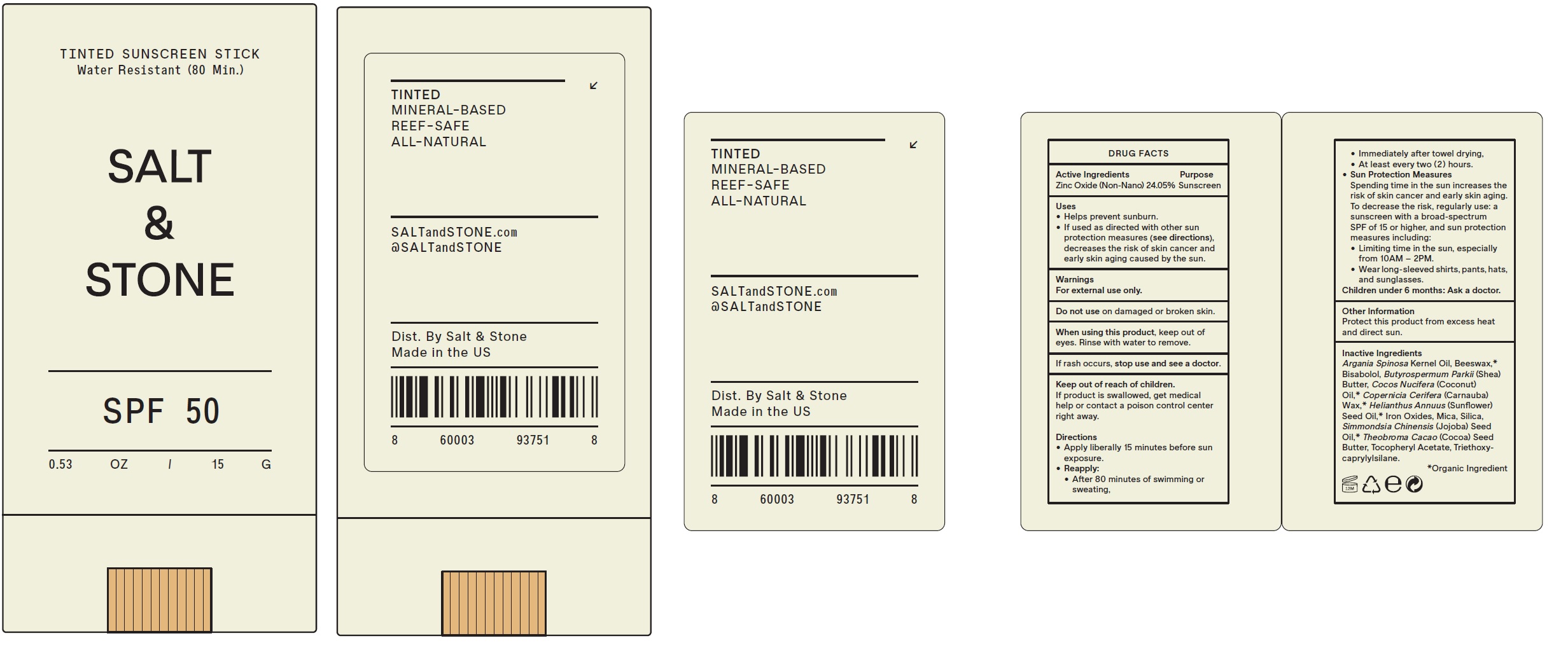
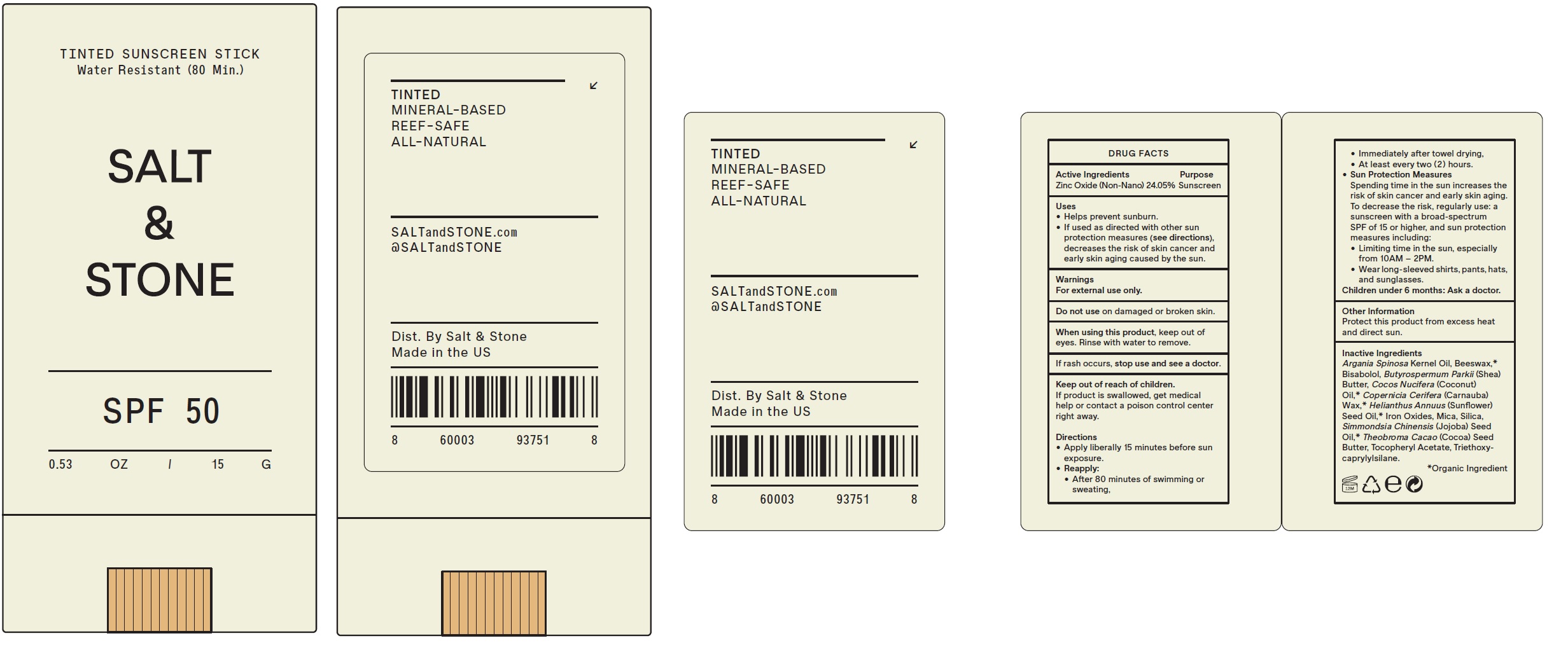
Label: SALT AND STONE TINTED SUNSCREEN SPF 50- zinc oxide stick
- NDC Code(s): 71585-138-00
- Packager: Salt and Stone LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- • After 80 minutes of swimming or sweating, • Immediately after towel drying, • At least every two ( 2 ) hours.
Reapply:
- Spending time in the sun increases the risk of skin cancer and early skin aging. To decrease the risk, regularly use: a sunscreen with a broad-spectrum SPF of 15 or higher, and sun protection measures including: • Limiting time in the sun, especially from 10AM – 2PM. • Wear long-sleeved shirts, pants, hats, and sunglasses.
Sun Protection Measures
- Children under 6 months: Ask a doctor.
- Other Information
-
Inactive Ingredients
Argania Spinosa Kernel Oil, Beeswax,* Bisabolol, Butyrospermum Parkii (Shea) Butter, Cocos Nucifera (Coconut) Oil,* Copernicia Cerifera (Carnauba) Wax,* Helianthus Annuus (Sunflower) Seed Oil,* Iron Oxides, Mica, Silica, Simmondsia Chinensis (Jojoba) Seed Oil,* Theobroma Cacao (Cocoa) Seed Butter, Tocopheryl Acetate, Triethoxycaprylylsilane.
- Package label
-
INGREDIENTS AND APPEARANCE
SALT AND STONE TINTED SUNSCREEN SPF 50
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71585-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 0.2405 g in 1 g Inactive Ingredients Ingredient Name Strength ARGAN OIL (UNII: 4V59G5UW9X) YELLOW WAX (UNII: 2ZA36H0S2V) LEVOMENOL (UNII: 24WE03BX2T) SHEA BUTTER (UNII: K49155WL9Y) COCONUT OIL (UNII: Q9L0O73W7L) CARNAUBA WAX (UNII: R12CBM0EIZ) SUNFLOWER OIL (UNII: 3W1JG795YI) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) JOJOBA OIL (UNII: 724GKU717M) COCOA BUTTER (UNII: 512OYT1CRR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71585-138-00 15 g in 1 APPLICATOR; Type 0: Not a Combination Product 11/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2020 Labeler - Salt and Stone LLC (080683697)