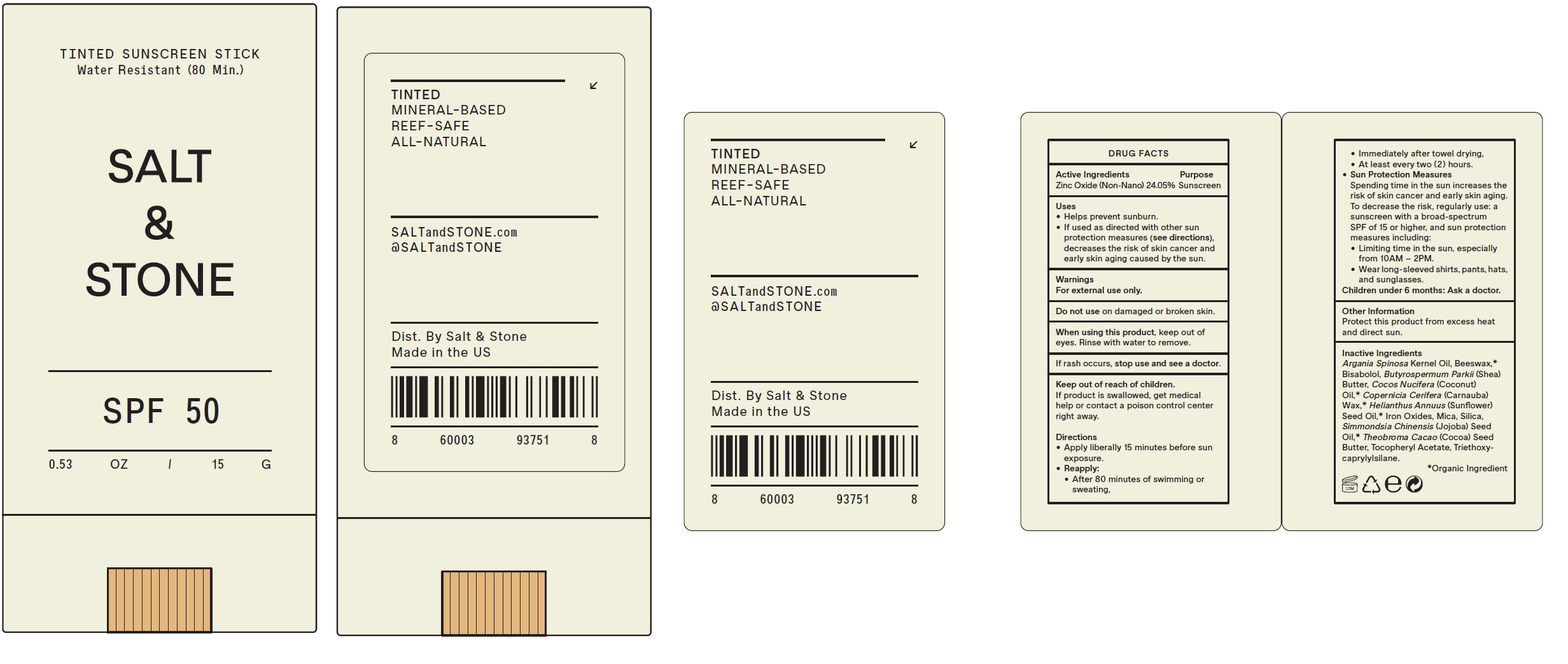
Uses
• Helps prevent sunburn. • If used as directed with other sun protection measures (see directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure.
- • After 80 minutes of swimming or sweating, • Immediately after towel drying, • At least every two ( 2 ) hours.
Reapply:
- Spending time in the sun increases the risk of skin cancer and early skin aging. To decrease the risk, regularly use: a sunscreen with a broad-spectrum SPF of 15 or higher, and sun protection measures including: • Limiting time in the sun, especially from 10AM – 2PM. • Wear long-sleeved shirts, pants, hats, and sunglasses.
Sun Protection Measures
- Children under 6 months: Ask a doctor.
Inactive Ingredients
Argania Spinosa Kernel Oil, Beeswax,* Bisabolol, Butyrospermum Parkii (Shea) Butter, Cocos Nucifera (Coconut) Oil,* Copernicia Cerifera (Carnauba) Wax,* Helianthus Annuus (Sunflower) Seed Oil,* Iron Oxides, Mica, Silica, Simmondsia Chinensis (Jojoba) Seed Oil,* Theobroma Cacao (Cocoa) Seed Butter, Tocopheryl Acetate, Triethoxycaprylylsilane.