Label: ENOXAPARIN SODIUM injection, solution
-
NDC Code(s):
11797-757-02,
11797-757-06,
11797-758-02,
11797-758-06, view more11797-759-02, 11797-759-06, 11797-760-02, 11797-760-06, 11797-761-02, 11797-761-06, 11797-762-02, 11797-762-06, 11797-763-02, 11797-763-06
- Packager: Italfarmaco SpA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
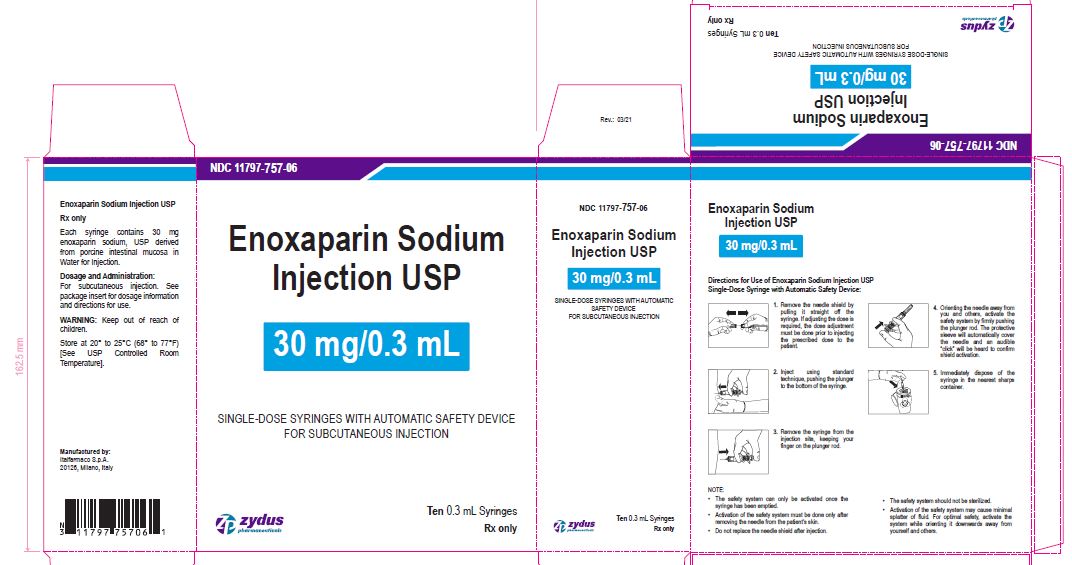
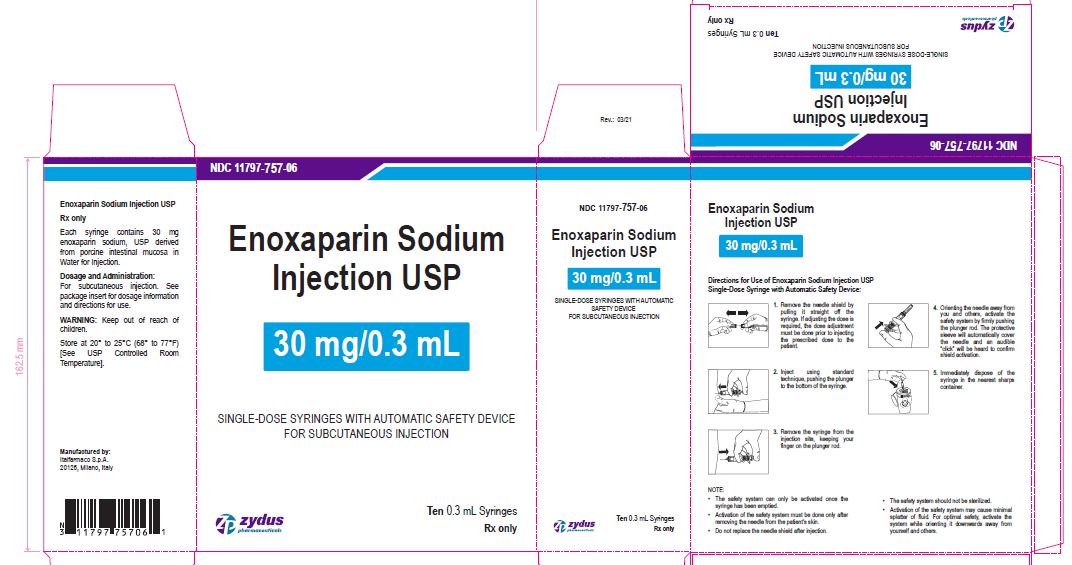
Enoxaparin Sodium Injection USP
30 mg/0.3 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.3 mL Syringes
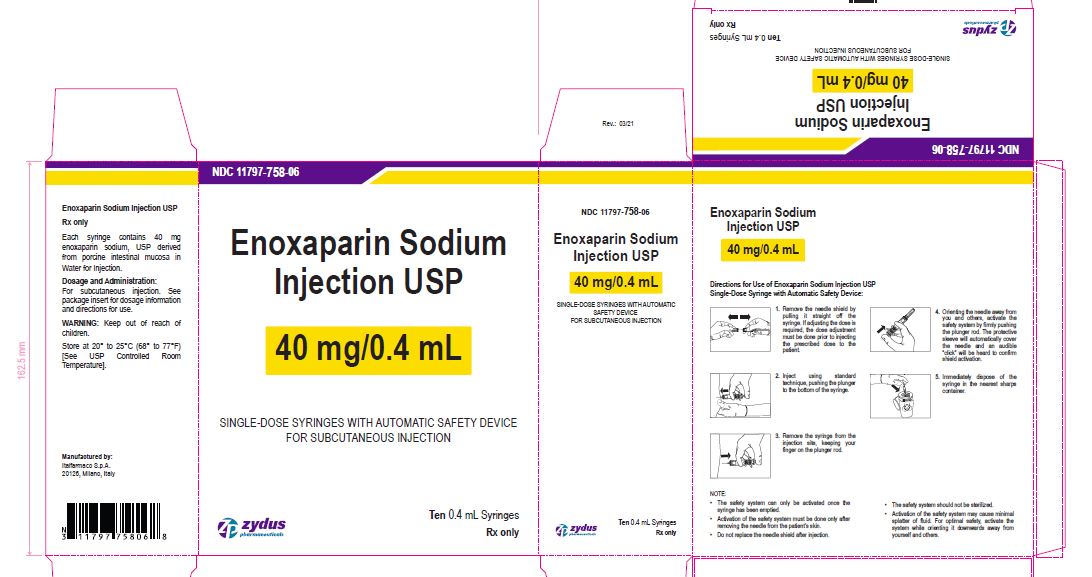
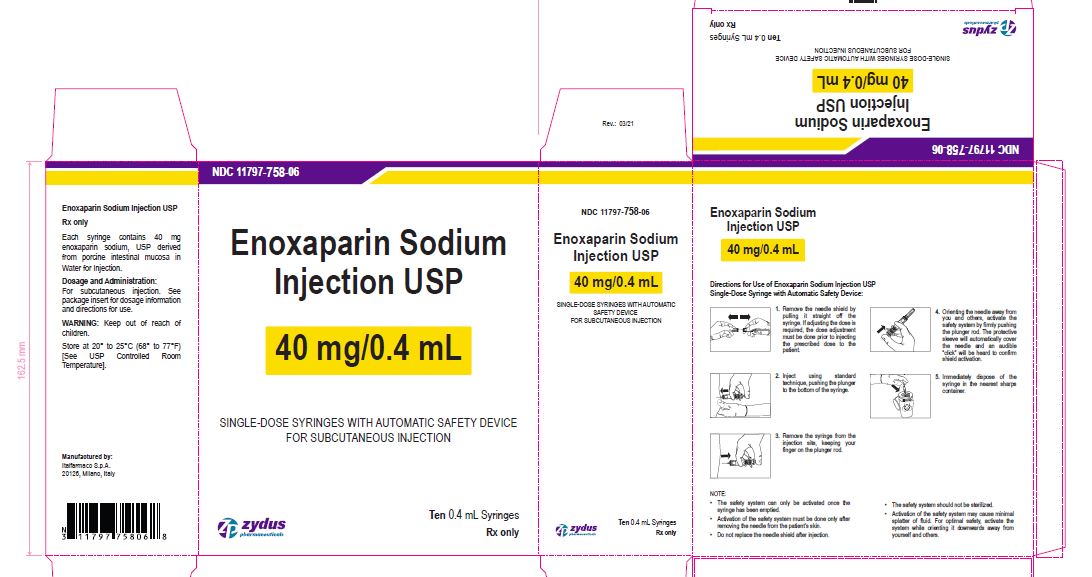
Enoxaparin Sodium Injection USP
40 mg/0.4 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.4 mL Syringes
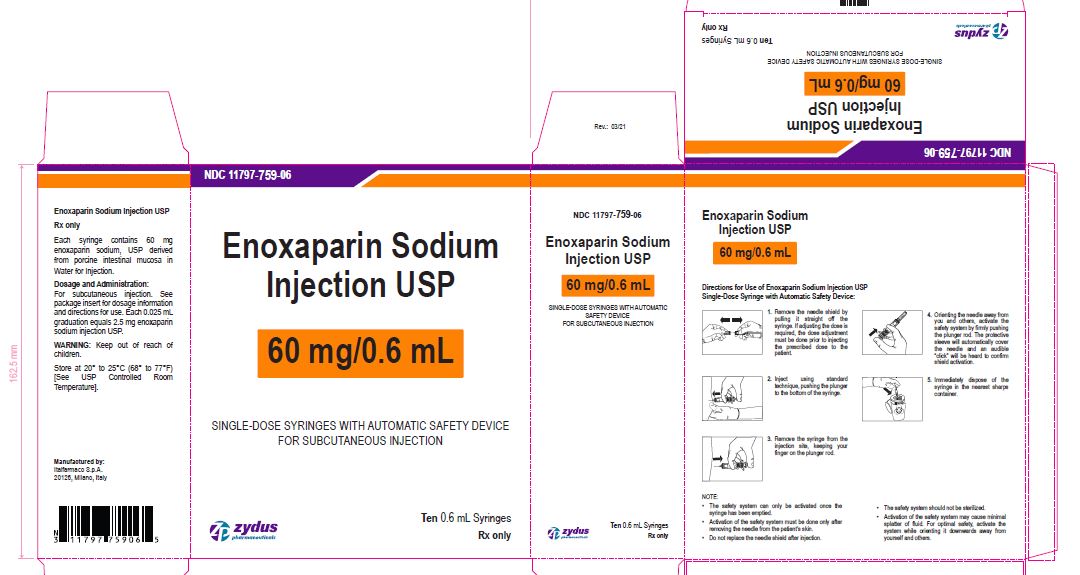
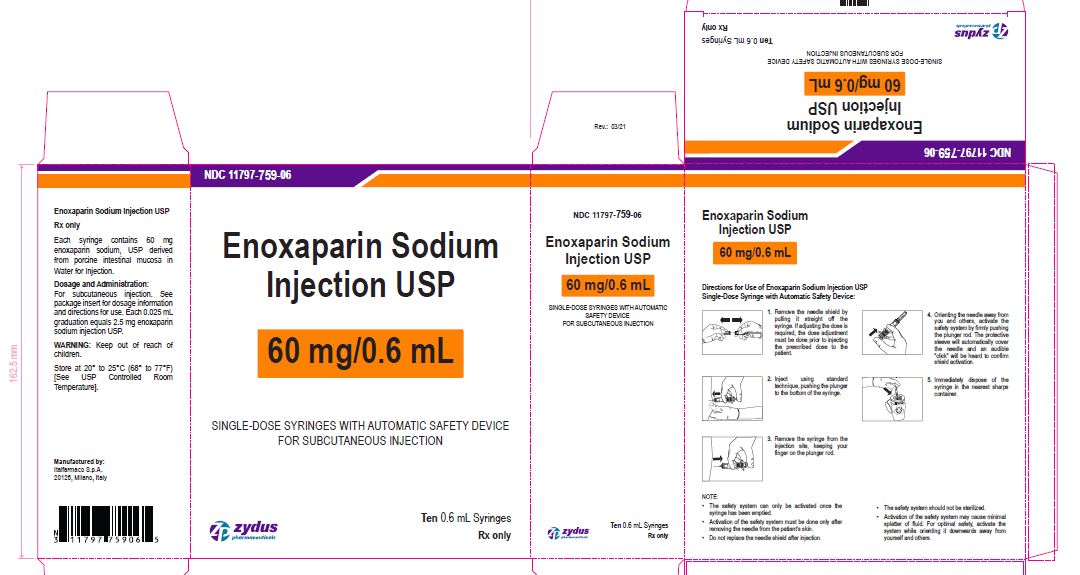
Enoxaparin Sodium Injection USP
60 mg/0.6 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.6 mL Syringes
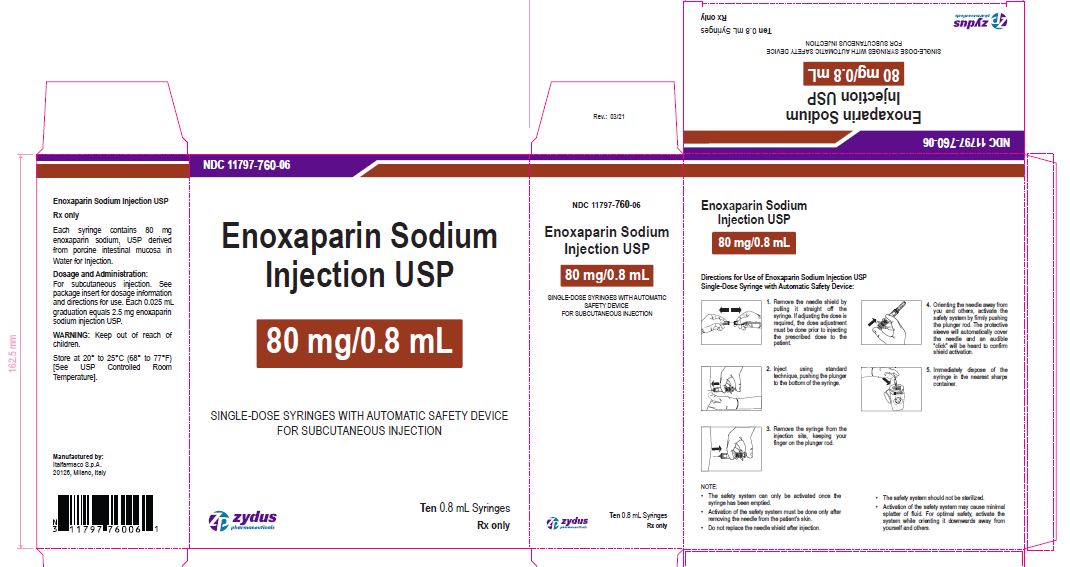
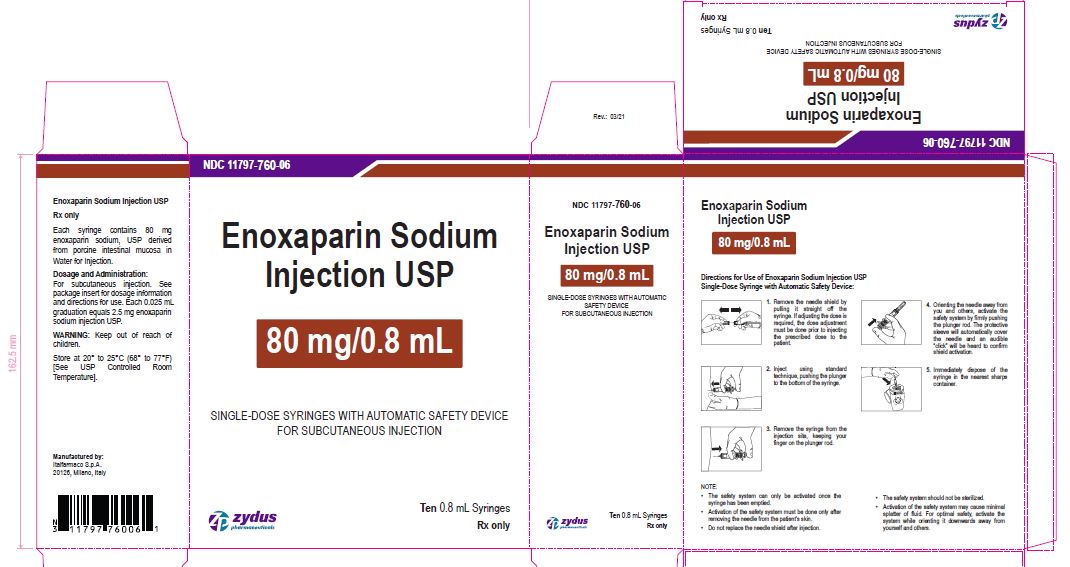
Enoxaparin Sodium Injection USP
80 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
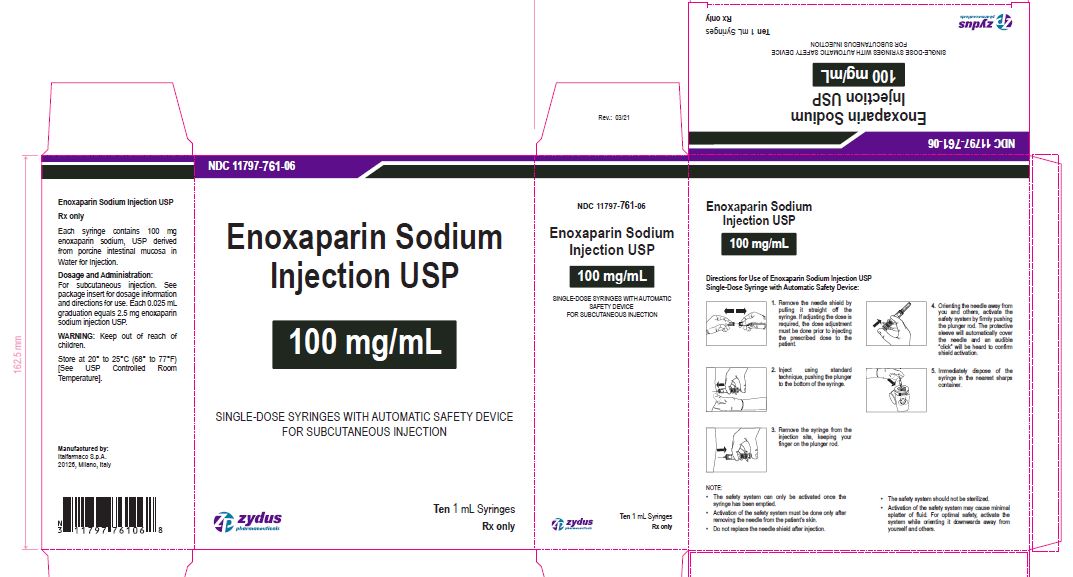
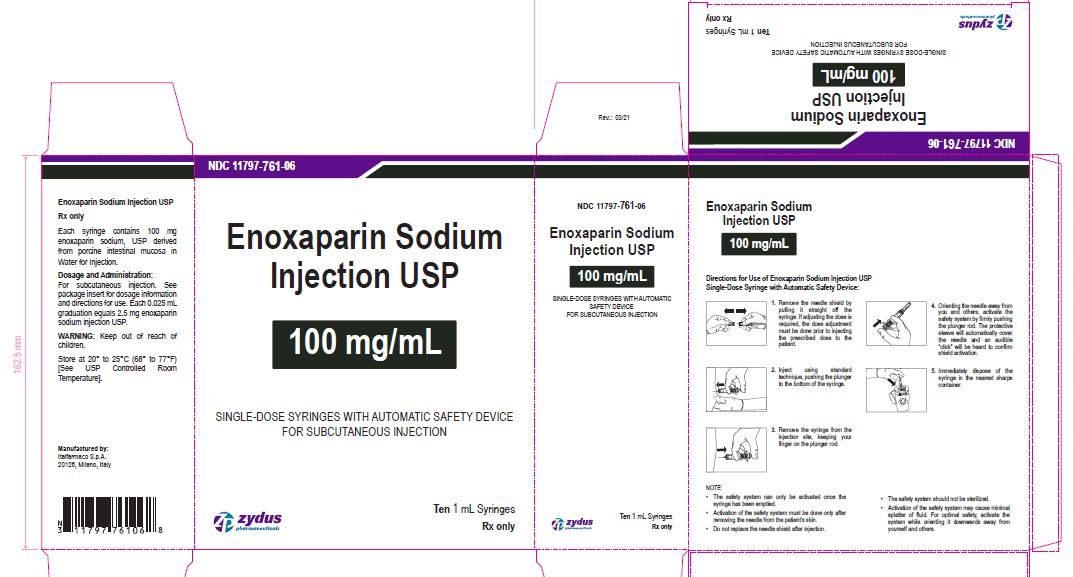
Enoxaparin Sodium Injection USP
100 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes
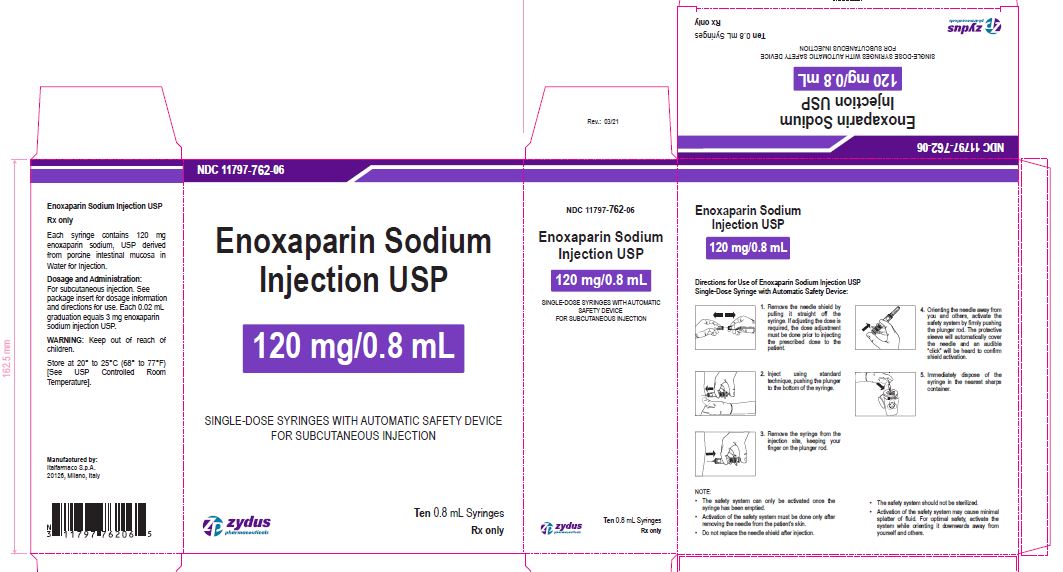
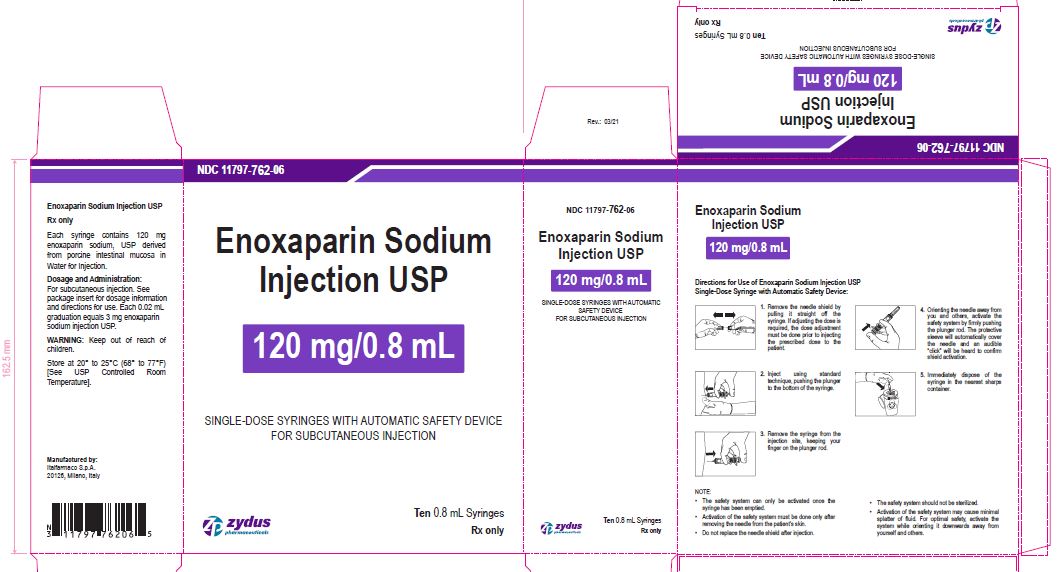
Enoxaparin Sodium Injection USP
120 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
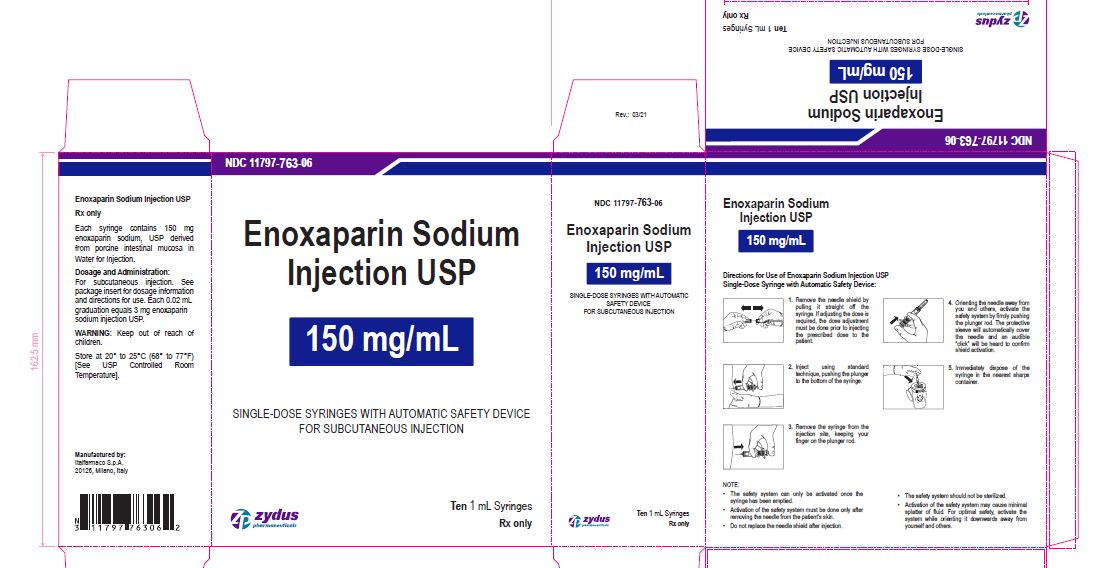
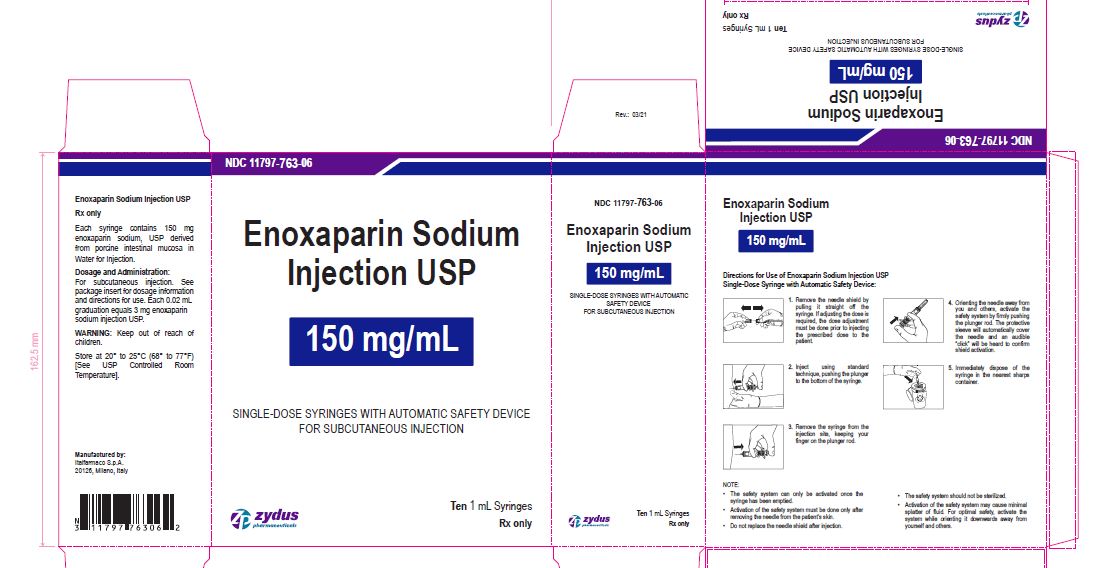
Enoxaparin Sodium Injection USP
150 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes
-
INGREDIENTS AND APPEARANCE
ENOXAPARIN SODIUM
enoxaparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11797-757 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENOXAPARIN SODIUM (UNII: 8NZ41MIK1O) (ENOXAPARIN - UNII:E47C0NF7LV) ENOXAPARIN SODIUM 30 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11797-757-06 10 in 1 CARTON 04/01/2021 1 NDC:11797-757-02 0.3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076726 04/01/2021 ENOXAPARIN SODIUM
enoxaparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11797-758 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENOXAPARIN SODIUM (UNII: 8NZ41MIK1O) (ENOXAPARIN - UNII:E47C0NF7LV) ENOXAPARIN SODIUM 40 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11797-758-06 10 in 1 CARTON 04/01/2021 1 NDC:11797-758-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076726 04/01/2021 ENOXAPARIN SODIUM
enoxaparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11797-759 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENOXAPARIN SODIUM (UNII: 8NZ41MIK1O) (ENOXAPARIN - UNII:E47C0NF7LV) ENOXAPARIN SODIUM 60 mg in 0.6 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11797-759-06 10 in 1 CARTON 04/01/2021 1 NDC:11797-759-02 0.6 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076726 04/01/2021 ENOXAPARIN SODIUM
enoxaparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11797-760 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENOXAPARIN SODIUM (UNII: 8NZ41MIK1O) (ENOXAPARIN - UNII:E47C0NF7LV) ENOXAPARIN SODIUM 80 mg in 0.8 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11797-760-06 10 in 1 CARTON 04/01/2021 1 NDC:11797-760-02 0.8 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076726 04/01/2021 ENOXAPARIN SODIUM
enoxaparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11797-761 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENOXAPARIN SODIUM (UNII: 8NZ41MIK1O) (ENOXAPARIN - UNII:E47C0NF7LV) ENOXAPARIN SODIUM 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11797-761-06 10 in 1 CARTON 04/01/2021 1 NDC:11797-761-02 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076726 04/01/2021 ENOXAPARIN SODIUM
enoxaparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11797-762 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENOXAPARIN SODIUM (UNII: 8NZ41MIK1O) (ENOXAPARIN - UNII:E47C0NF7LV) ENOXAPARIN SODIUM 120 mg in 0.8 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11797-762-06 10 in 1 CARTON 04/01/2021 1 NDC:11797-762-02 0.8 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076726 04/01/2021 ENOXAPARIN SODIUM
enoxaparin sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:11797-763 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENOXAPARIN SODIUM (UNII: 8NZ41MIK1O) (ENOXAPARIN - UNII:E47C0NF7LV) ENOXAPARIN SODIUM 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11797-763-06 10 in 1 CARTON 04/01/2021 1 NDC:11797-763-02 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076726 04/01/2021 Labeler - Italfarmaco SpA (428179329) Establishment Name Address ID/FEI Business Operations Italfarmaco SpA 428179329 MANUFACTURE(11797-757, 11797-758, 11797-759, 11797-760, 11797-761, 11797-762, 11797-763)