PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
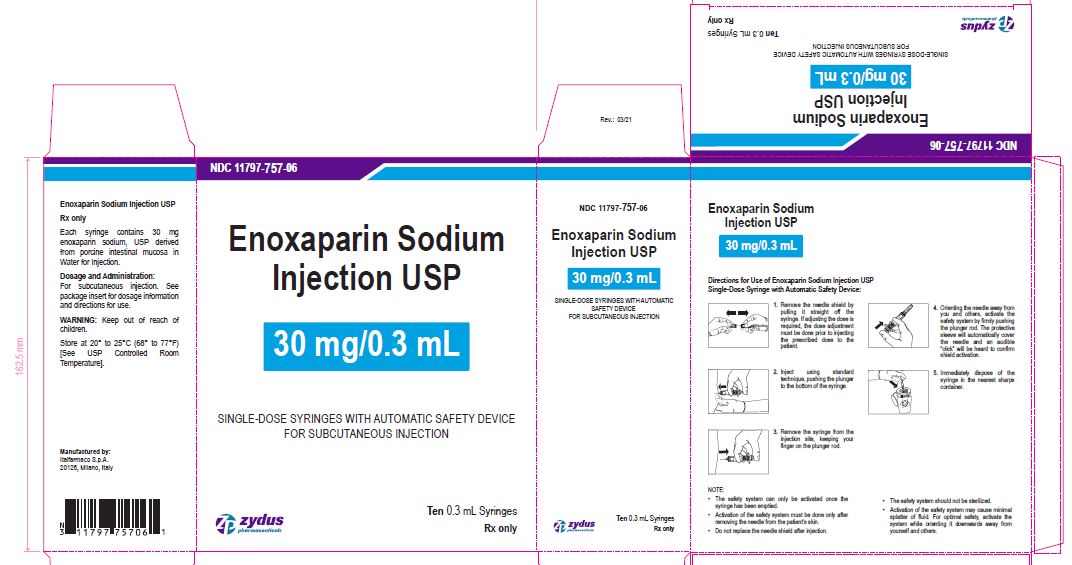
Enoxaparin Sodium Injection USP
30 mg/0.3 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.3 mL Syringes
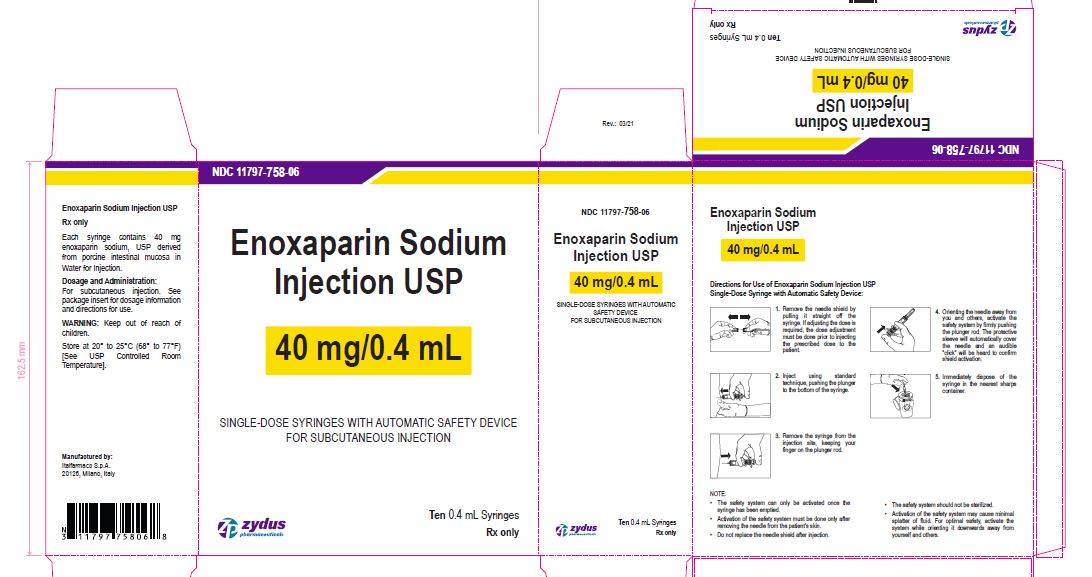
Enoxaparin Sodium Injection USP
40 mg/0.4 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.4 mL Syringes
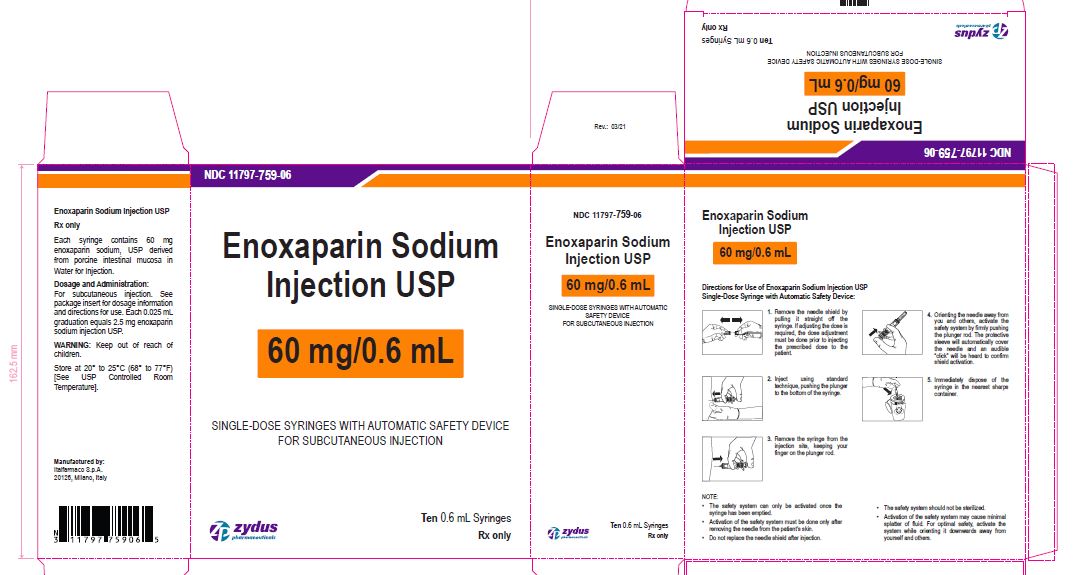
Enoxaparin Sodium Injection USP
60 mg/0.6 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.6 mL Syringes
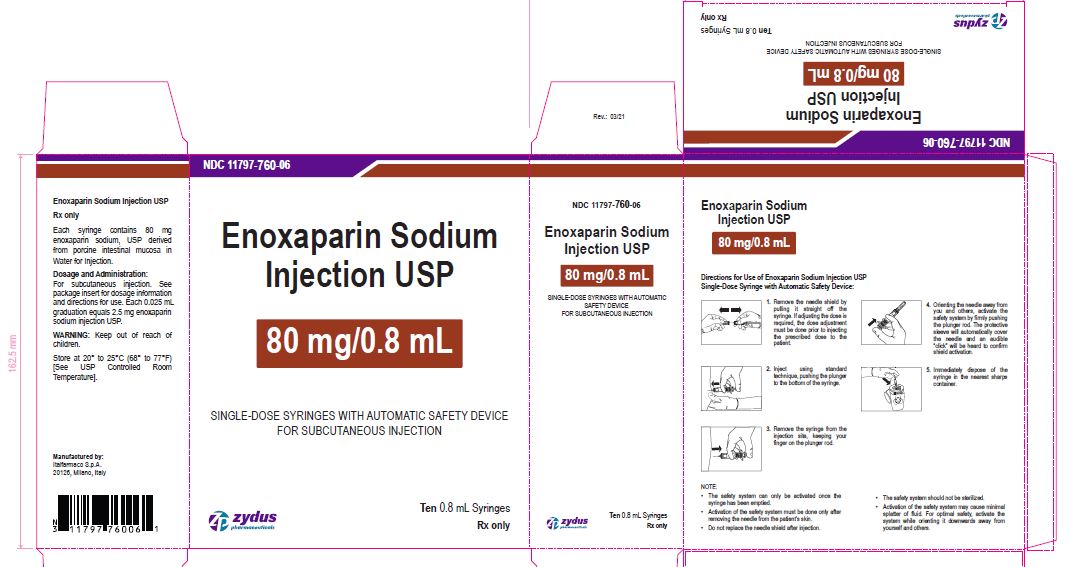
Enoxaparin Sodium Injection USP
80 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
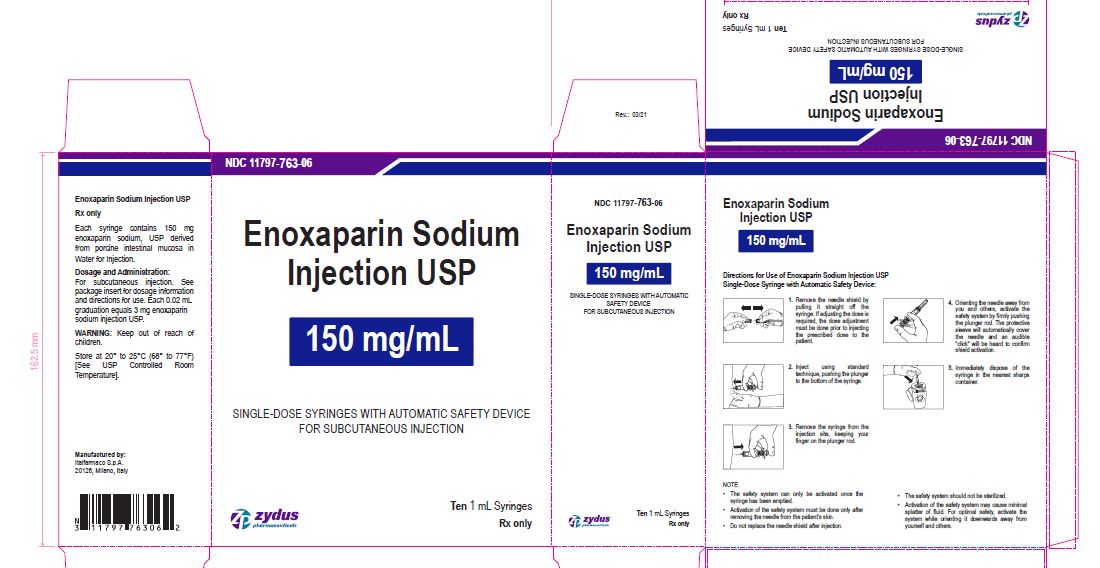
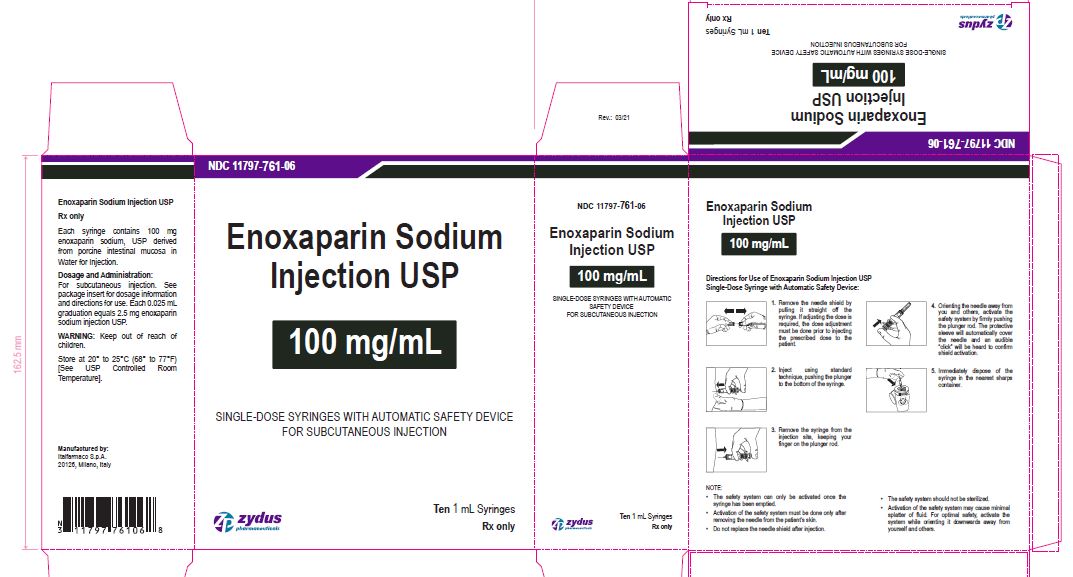
Enoxaparin Sodium Injection USP
100 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes
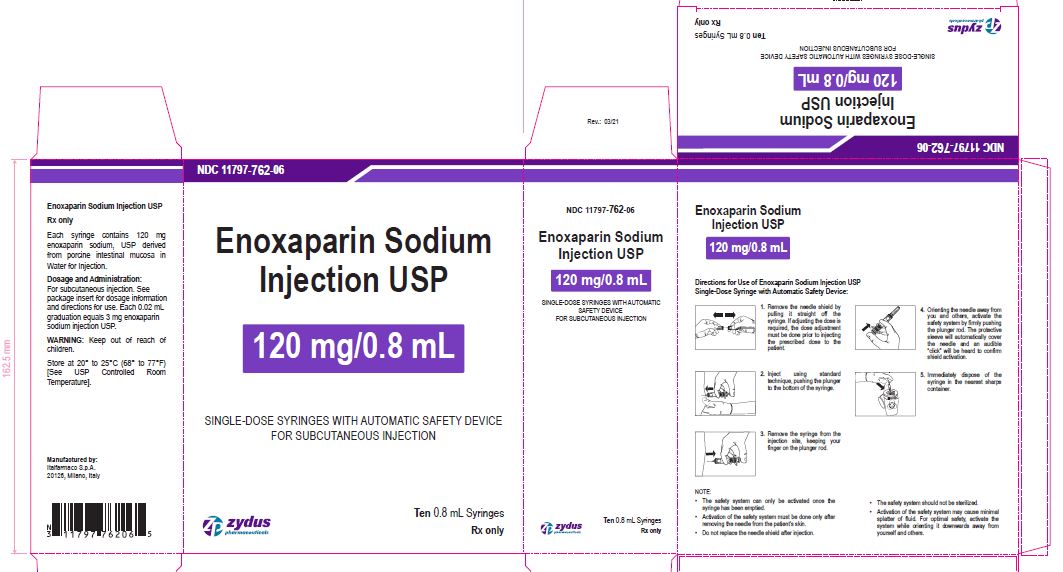
Enoxaparin Sodium Injection USP
120 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
Enoxaparin Sodium Injection USP
150 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes