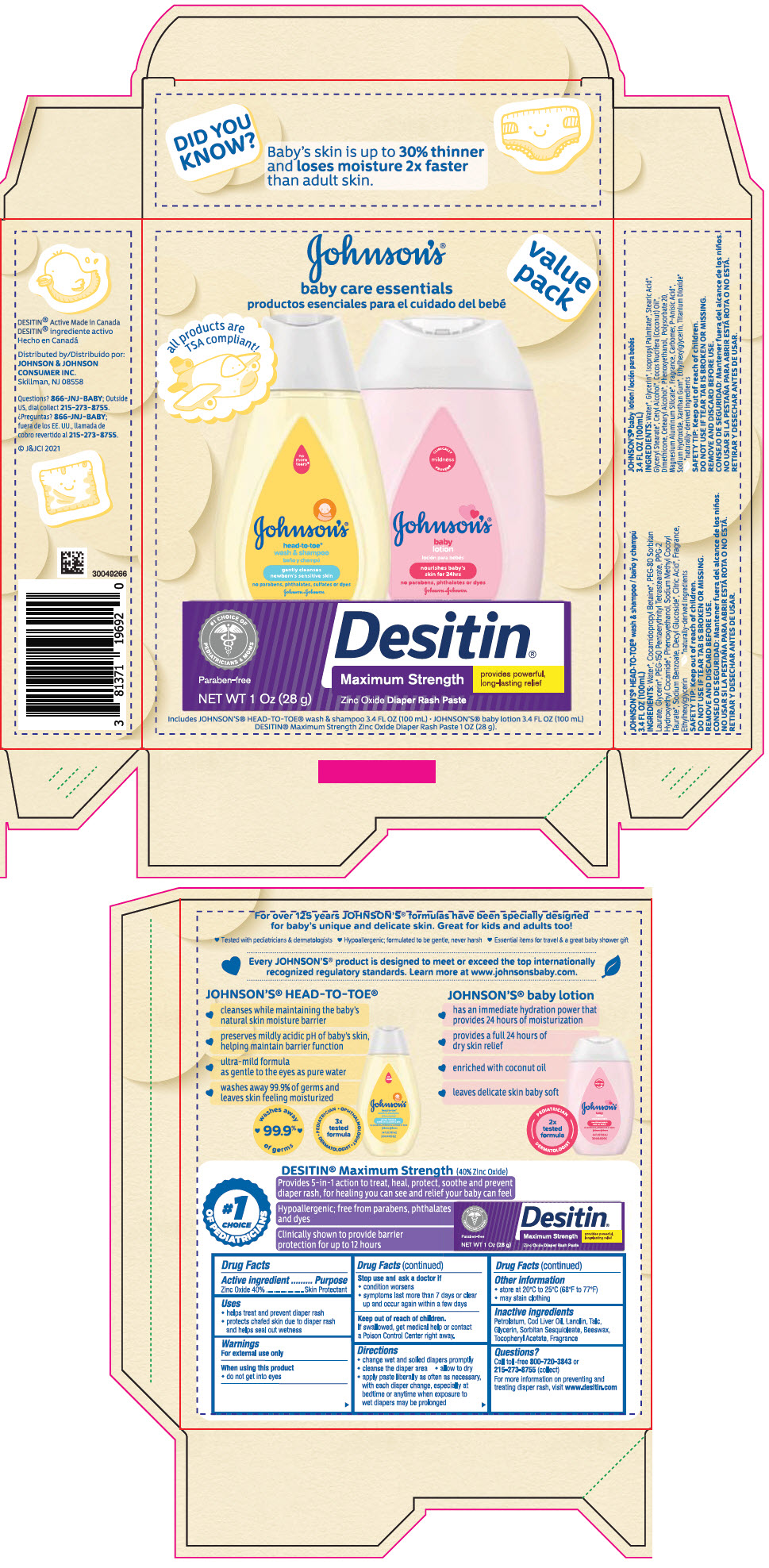
Label: JOHNSONS BABY CARE ESSENTIALS GIFT SET- zinc oxide kit
- NDC Code(s): 69968-0061-1, 69968-0713-9
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Package
JOHNSON'S ®
baby care essentials
productos esenciales para el cuidado del bebévalue
packall products are
TSA compliant!Includes JOHNSON'S ® HEAD-TO TOE ® wash and shampoo 3.4 FL OZ (100 mL) ● JOHNSON'S ® baby lotion 3.4 FL OZ (100 mL)
DESITIN ® Maximum Strength Zinc Oxide Diaper Rash Paste 1 OZ (28 g)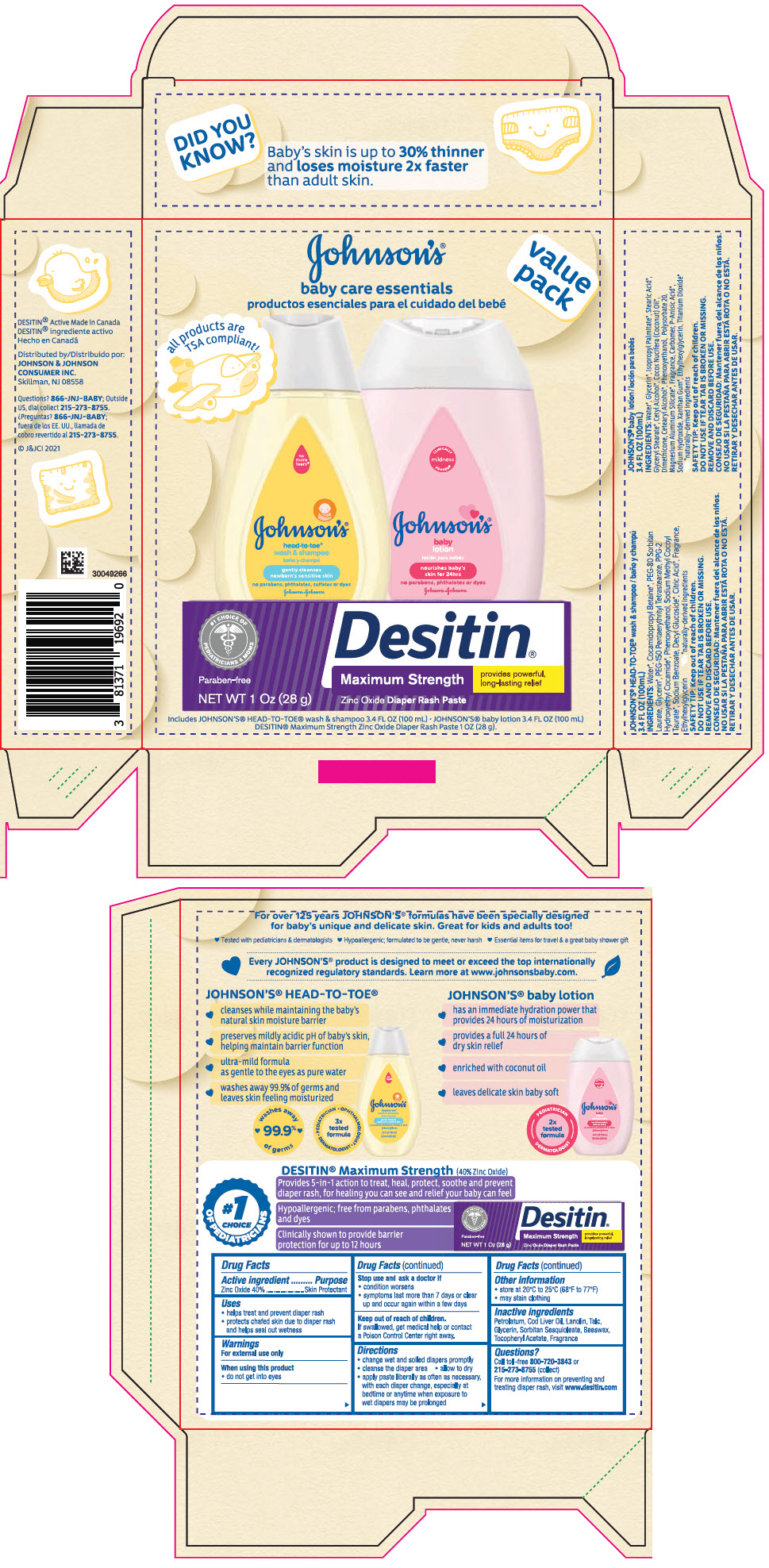
-
INGREDIENTS AND APPEARANCE
JOHNSONS BABY CARE ESSENTIALS GIFT SET
zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0713 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0713-9 1 in 1 PACKAGE 07/02/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 28 g Part 2 1 BOTTLE 100 mL Part 3 1 BOTTLE 100 mL Part 1 of 3 DESITIN MAXIMUM STRENGTH DIAPER RASH
zinc oxide pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 400 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) COD LIVER OIL (UNII: BBL281NWFG) LANOLIN (UNII: 7EV65EAW6H) TALC (UNII: 7SEV7J4R1U) GLYCERIN (UNII: PDC6A3C0OX) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) YELLOW WAX (UNII: 2ZA36H0S2V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0061-1 1 in 1 CARTON 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/16/2015 Part 2 of 3 JOHNSONS HEAD TO TOE WASH
baby shampoos [baby products] liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR PEG-80 SORBITAN LAURATE (UNII: 239B50Y732) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) INGR PPG-2 HYDROXYETHYL COCAMIDE (UNII: 34N07GUJ3X) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR DECYL GLUCOSIDE (UNII: Z17H97EA6Y) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 07/09/2018 Part 3 of 3 JOHNSONS BABY
lotions, oils, powders, and creams [baby products] lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) INGR CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) INGR P-ANISIC ACID (UNII: 4SB6Y7DMM3) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR TITANIUM DIOXIDE (UNII: 15FIX9V2JP) INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) INGR STEARIC ACID (UNII: 4ELV7Z65AP) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 07/09/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 07/02/2021 Labeler - Kenvue Brands LLC (118772437)