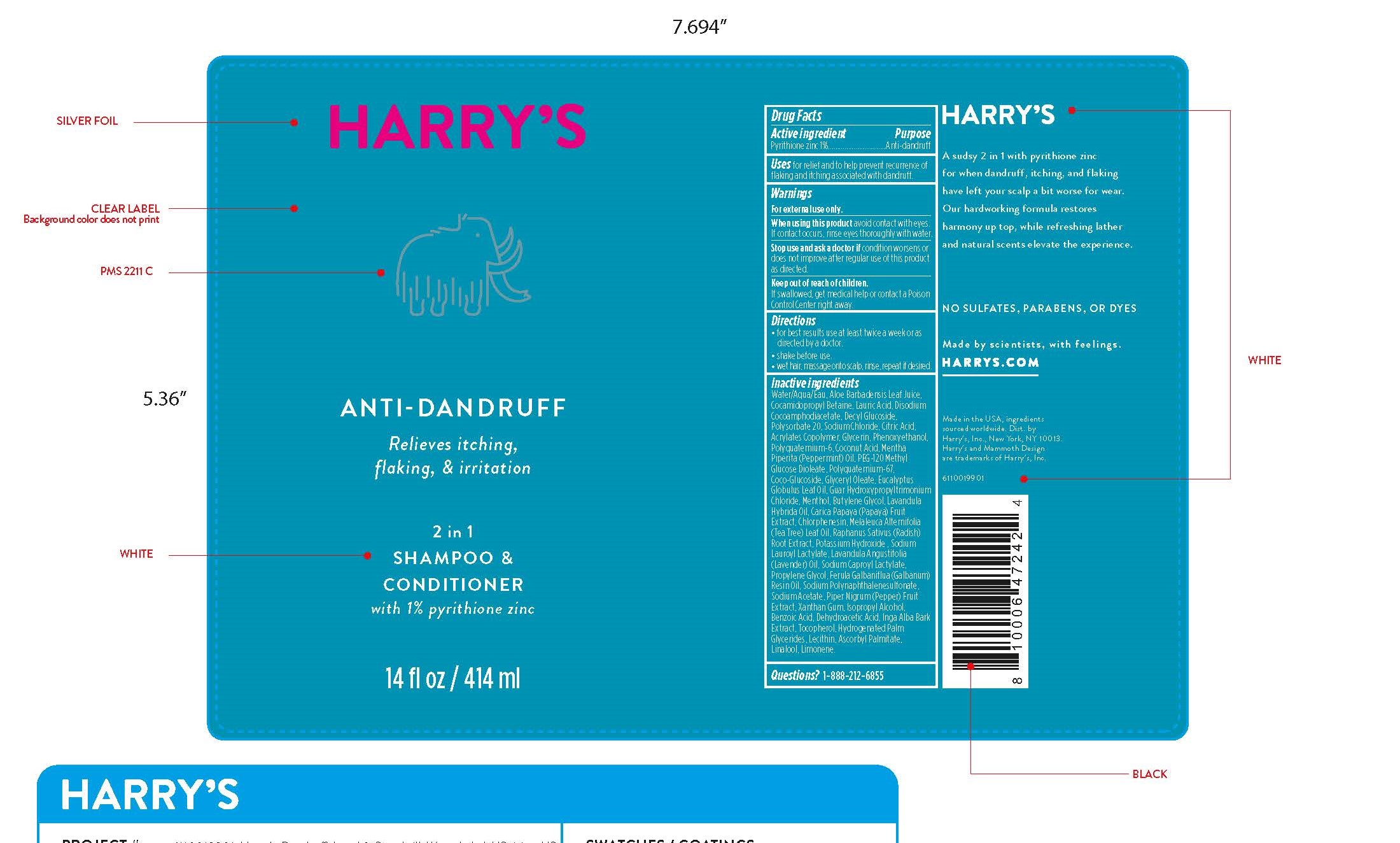
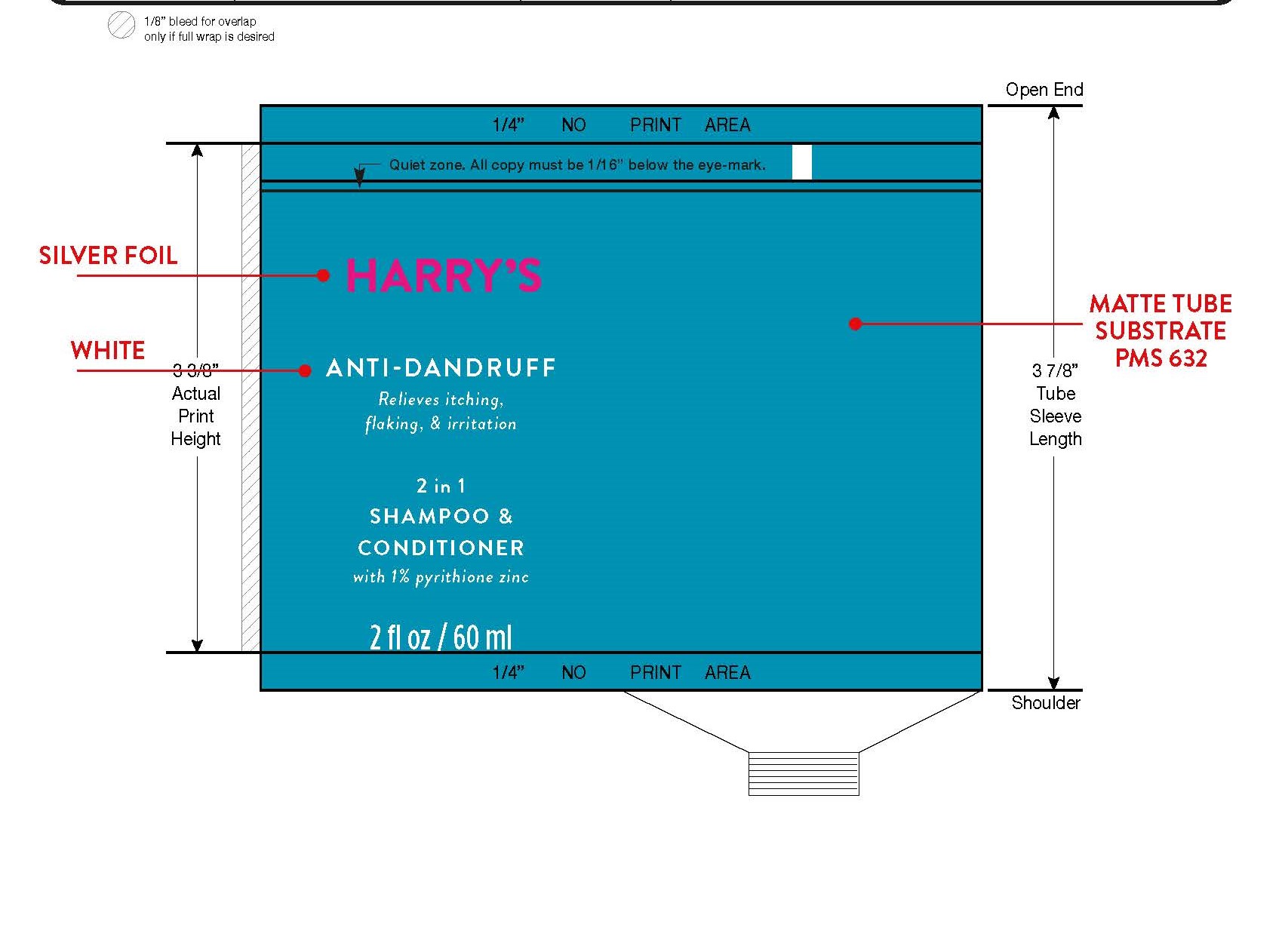
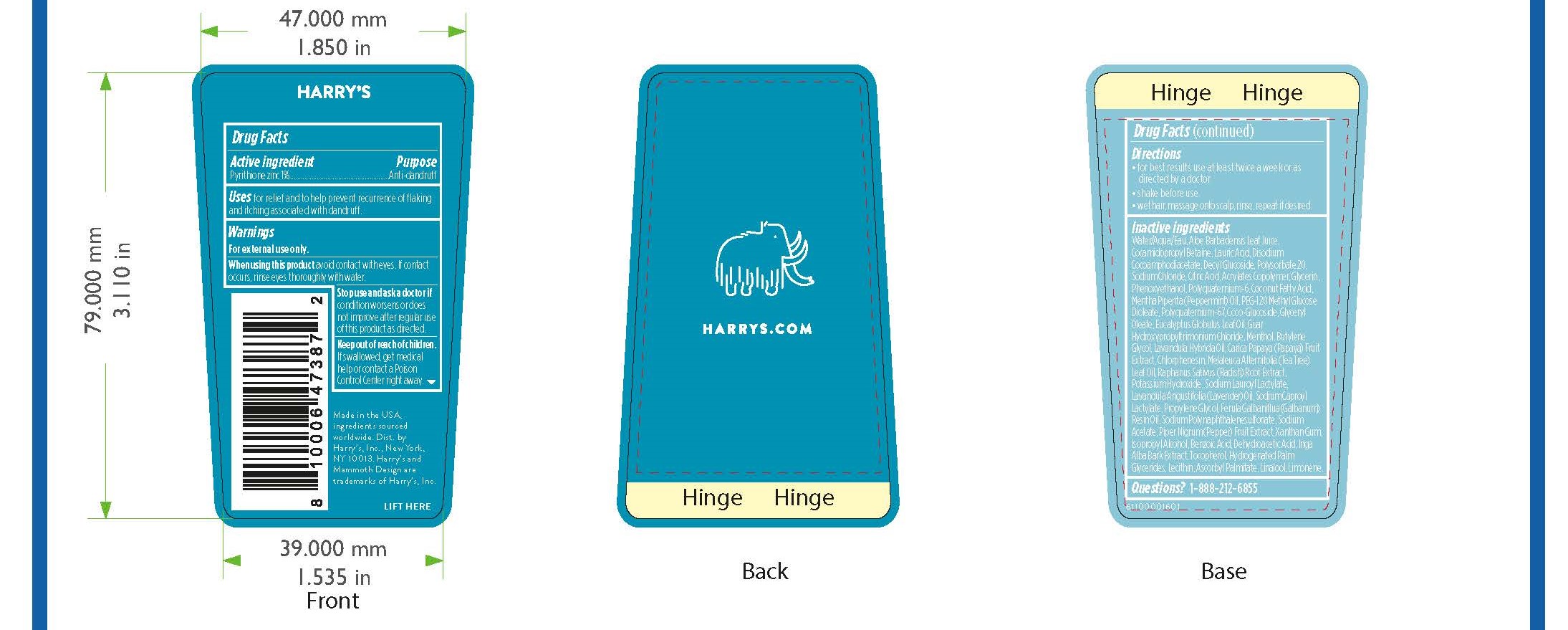
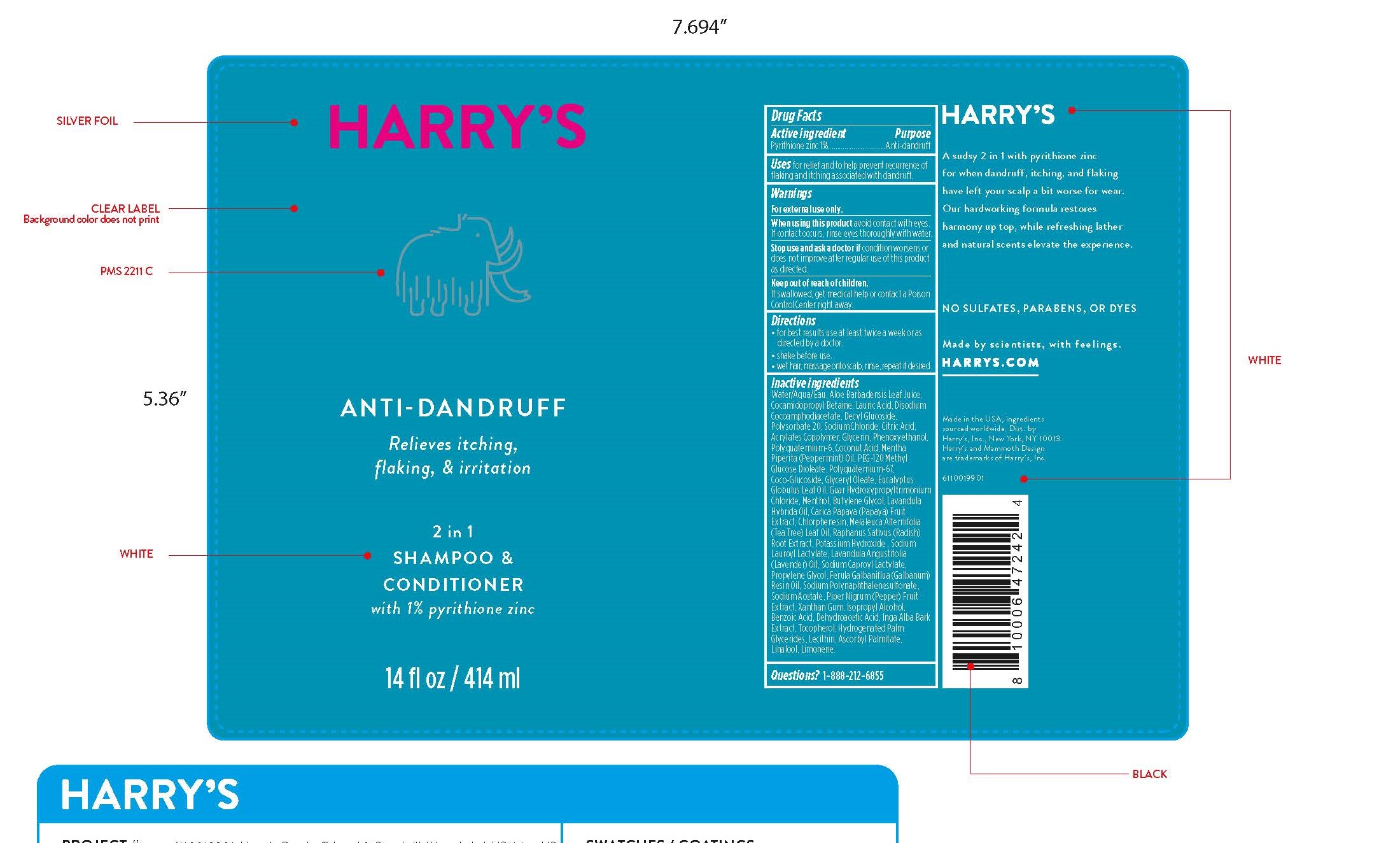
Label: ZINC PYRITHIONE shampoo
- NDC Code(s): 70533-003-02, 70533-003-14
- Packager: Harry's Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Water/Aqua/Eau, Aloe Barbadensis Leaf Juice, Cocamidopropyl Betaine, Lauric Acid, Disodium Cocoamphodiacetate, Decyl Glucoside, Polysorbate 20, Sodium Chloride, Citric Acid, Acrylates Copolymer, Glycerin, Phenoxyethanol, Polyquaternium-6, Coconut Acid, Mentha Piperita (Peppermint) Oil, PEG-120 Methyl Glucose Dioleate, Polyquaternium-67, Coco-Glucoside, Glyceryl Oleate, Eucalyptus Globulus Leaf Oil, Guar Hydroxypropyltrimonium Chloride, Menthol, Butylene Glycol, Lavandula Hybrida Oil, Carica Papaya (Papaya) Fruit Extract, Chlorphenesin, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Raphanus Sativus (Radish)
Root Extract, Potassium Hydroxide , Sodium Lauroyl Lactylate, Lavandula Angustifolia (Lavender) Oil, Sodium Caproyl Lactylate,
Propylene Glycol, Ferula Galbaniflua (Galbanum) Resin Oil, Sodium Polynaphthalenesulfonate, Sodium Acetate, Piper Nigrum (Pepper) Fruit Extract, Xanthan Gum, Isopropyl Alcohol, Benzoic Acid, Dehydroacetic Acid, Inga Alba Bark Extract, Tocopherol, Hydrogenated Palm Glycerides, Lecithin, Ascorbyl Palmitate, Linalool, Limonene. - QUESTIONS
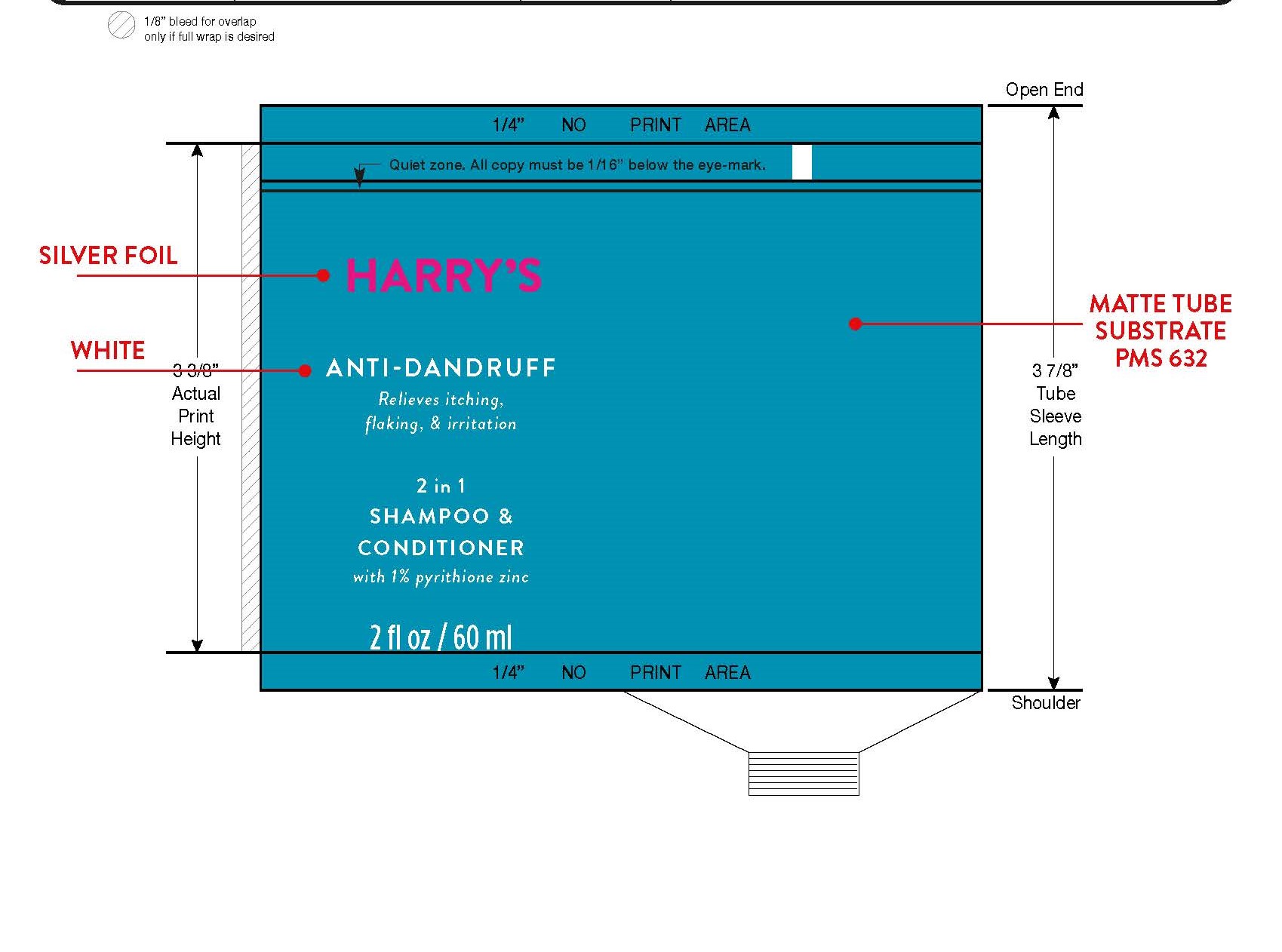
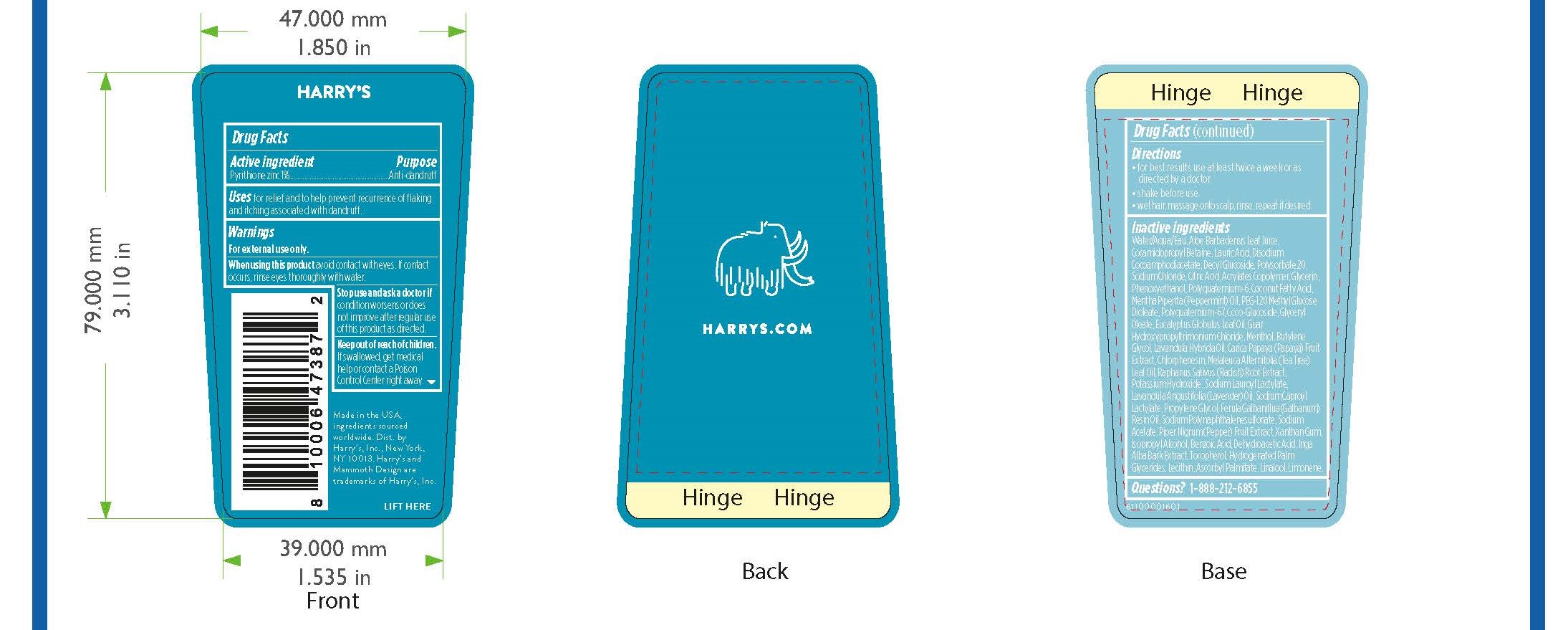
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZINC PYRITHIONE
zinc pyrithione shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70533-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength DECYL GLUCOSIDE (UNII: Z17H97EA6Y) POLYQUATERNIUM-6 (15000 MW) (UNII: YFL33X52PX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LIMONENE, (+/-)- (UNII: 9MC3I34447) FORMALDEHYDE/SODIUM NAPHTHALENESULFONATE COPOLYMER (3000 MW) (UNII: 90D834OZUI) ALOE (UNII: V5VD430YW9) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PAPAYA (UNII: KU94FIY6JB) LAURIC ACID (UNII: 1160N9NU9U) INGA ALBA BARK (UNII: 942K688993) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) POLYQUATERNIUM-10 (400 CPS AT 2%) (UNII: HB1401PQFS) SODIUM CHLORIDE (UNII: 451W47IQ8X) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAVANDIN OIL (UNII: 9RES347CKG) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCO GLUCOSIDE (UNII: ICS790225B) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) RACEMENTHOL (UNII: YS08XHA860) CHLORPHENESIN (UNII: I670DAL4SZ) TEA TREE OIL (UNII: VIF565UC2G) GALBANUM OIL (UNII: 211UF7M8N1) DEHYDROACETIC ACID (UNII: 2KAG279R6R) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (4500 MPA.S) (UNII: T967IEU43C) GLYCERYL OLEATE (UNII: 4PC054V79P) EUCALYPTUS OIL (UNII: 2R04ONI662) LINALOOL, (+/-)- (UNII: D81QY6I88E) SODIUM ACETATE ANHYDROUS (UNII: NVG71ZZ7P0) WHITE PEPPER (UNII: M29DW54Q9E) RAPHANUS SATIVUS VAR. CAUDATUS ROOT (UNII: 1D6EC6L1NP) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) COCONUT ACID (UNII: 40U37V505D) PEPPERMINT OIL (UNII: AV092KU4JH) ASCORBYL PALMITATE (UNII: QN83US2B0N) XANTHAN GUM (UNII: TTV12P4NEE) BENZOIC ACID (UNII: 8SKN0B0MIM) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) LAVENDER OIL (UNII: ZBP1YXW0H8) SODIUM CAPROYL LACTYLATE (UNII: 87WR3BHC09) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70533-003-14 414 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2020 10/01/2026 2 NDC:70533-003-02 59 mL in 1 TUBE; Type 0: Not a Combination Product 10/01/2020 10/01/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 10/01/2020 10/01/2026 Labeler - Harry's Inc. (079239206)