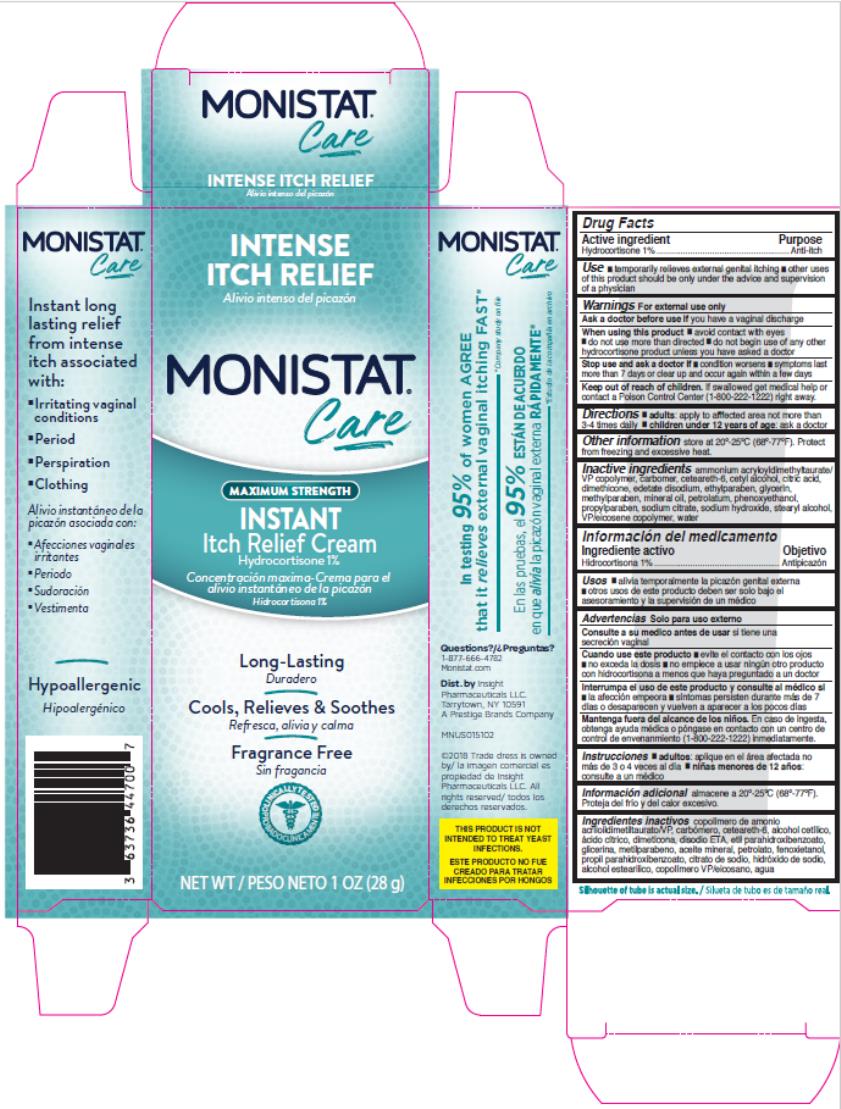
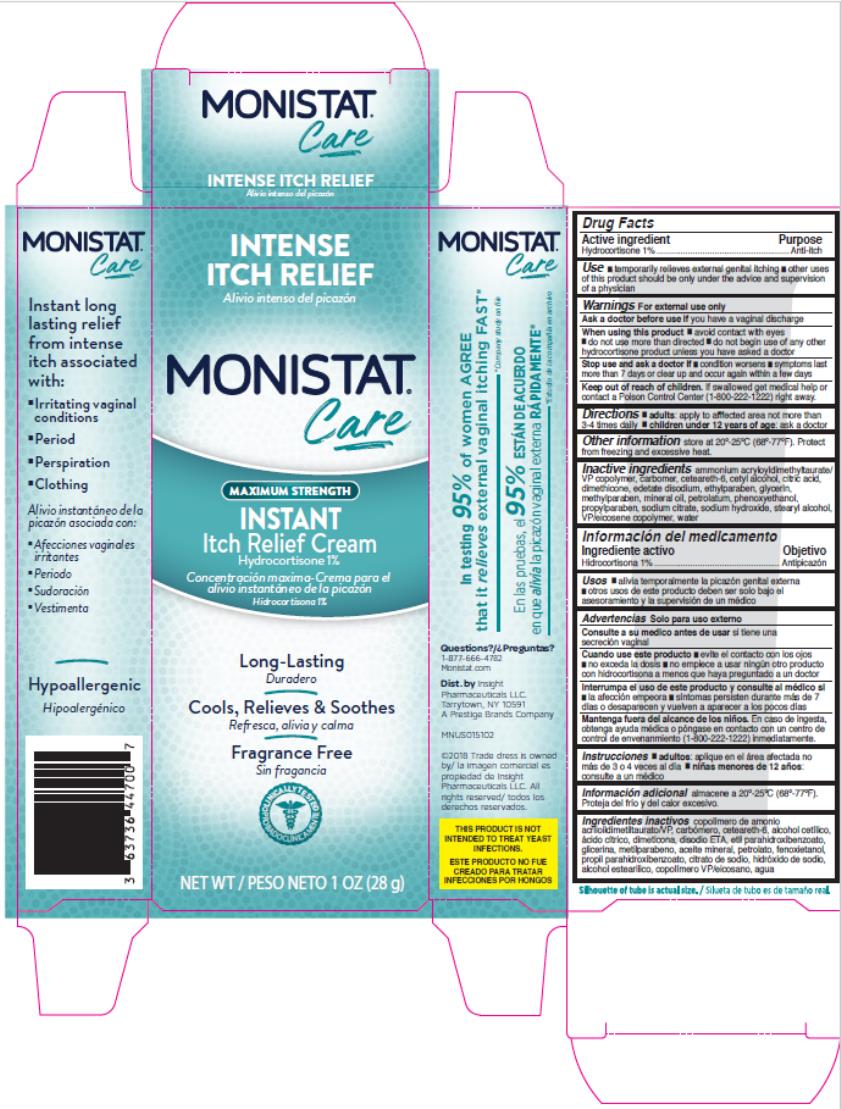
Label: MONISTAT COMPLETE CARE INSTANT ITCH RELIEF- hydrocortisone cream
- NDC Code(s): 63736-029-01
- Packager: Insight Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
- avoid contact with eyes
- do not use more than directed
- do not begin use of any other hydrocortisone product unless you have asked a doctor
- avoid contact with eyes
- Directions
- Other Information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MONISTAT COMPLETE CARE INSTANT ITCH RELIEF
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63736-029 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CARBOMER HOMOPOLYMER TYPE A (UNII: F68VH75CJC) CETEARETH-6 (UNII: 2RJS3559D3) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLPARABEN (UNII: 14255EXE39) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63736-029-01 1 in 1 CARTON 11/15/2013 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/15/2013 Labeler - Insight Pharmaceuticals LLC (055665422)