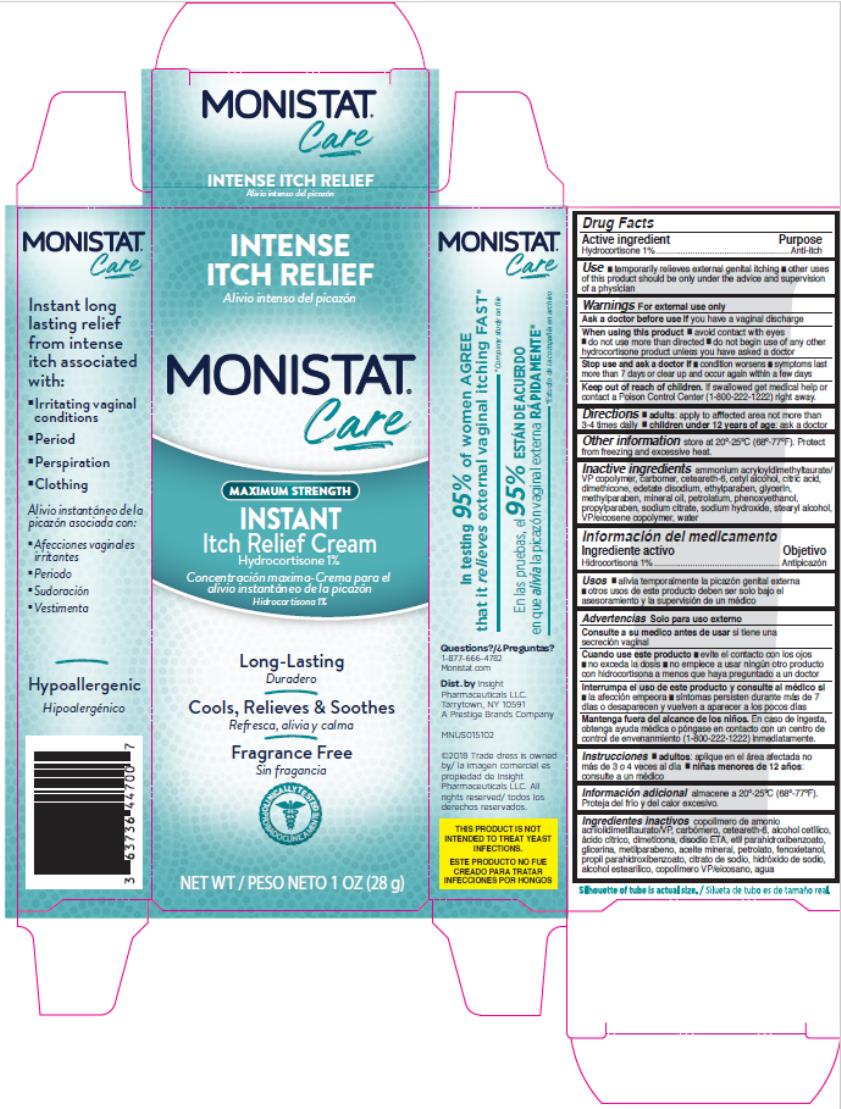
MONISTAT COMPLETE CARE INSTANT ITCH RELIEF- hydrocortisone cream
Insight Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Hydrocortisone 1%
Uses
- temporarily relieves external genital itching
- other uses of this product should only be under the advice and supervision of a doctor
Warnings
For external use only
Ask a doctor before use if
you have a vaginal discharge
When using this product
- avoid contact with eyes
- do not use more than directed
- do not begin use of any other hydrocortisone product unless you have asked a doctor
Stop use and ask a doctor if
- condition worsens
- symptoms last for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
-
adults: apply to affected area not more than 3-4 times daily
-
children under 12 years of age: ask a doctor
Other Information
- store at 20°-25°C (68°-77°F). Protect from freezing and excessive heat.
Inactive ingredients
ammonium acryloyldimethyltaurate/VP copolymer, carbomer, ceteareth-6, cetyl alcohol, citric acid, dimethicone, edetate disodium, ethylparaben, glycerin, methylparaben, mineral oil, petrolatum, phenoxyethanol, propylparaben, sodium citrate, sodium hydroxide, stearyl alcohol, VP/eicosene copolymer, water
Questions?
1-877-666-4782 Monistat.com
Dist. by Insight Pharmaceuticals, LLC.
Tarrytown, NY 10591
A Prestige Brands Company
PRINCIPAL DISPLAY PANEL
MONISTAT®
Care
Itch Relief Cream
Hydrocortisone 1%
NEW WT 1 OZ (28 g)