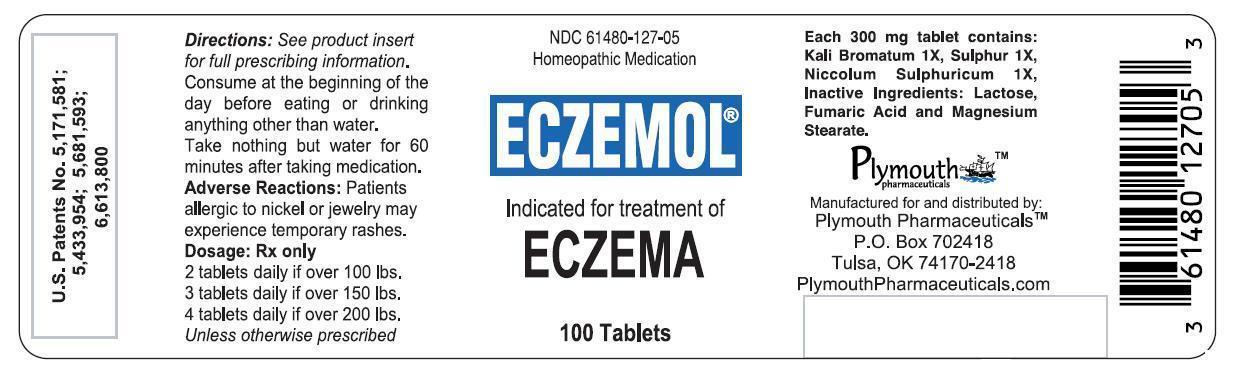
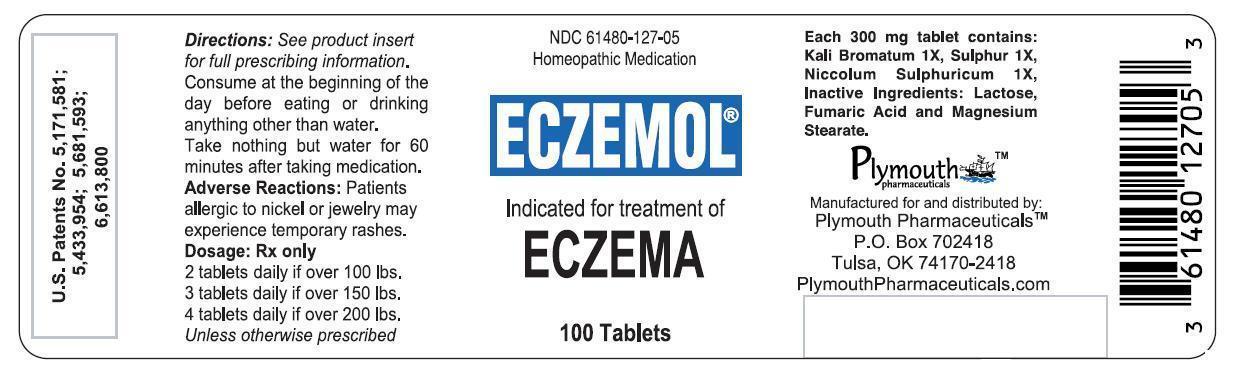
Label: ECZEMOL- potassium bromide, nickel sulfate, and sulfur tablet
- NDC Code(s): 61480-127-05
- Packager: PLYMOUTH HEALTHCARE PRODUCTS LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- CAUTION
-
DESCRIPTION
ECZEMOL ®is a biochemical homeopathic medication indicated for the treatment of eczema.27,29The active ingredients in each ECZEMOL ®tablet consist of the following: Potassium Bromide (Kali Bromatum) 1X, Sulphur 1X, and Nickel Sulphate (Niccolum Sulphuricum) 1X. These drug ingredients are listed in the Homoeopathic Pharmacopoeia of the United States (HPUS). 1
Inactive ingredients: Lactose, Fumaric Acid, and Magnesium Stearate.
Pharmacological class:Homeopathic drug.
Dosage form:Oral 300 mg scored tablet. May be swallowed whole, chewed or dissolved in the mouth and swallowed.
-
CLINICAL PHARMACOLOGY
The active ingredients in ECZEMOL ®are simple biochemical compounds. The exact mechanism of action is unknown; however, it is believed ECZEMOL ®addresses a primary genetic biochemical defect.
POTASSIUM BROMIDEdissolves and dissociates in the digestive tract into its ionic constituents. Each tablet contains approximately 15 mg of bromide (calculated). Ionic bromide is rapidly and completely absorbed from the intestine and distributed almost exclusively in the extracellular fluids. 7,8Bromide is eliminated by the kidneys and the elimination half-life is 11-12 days. "Once a day" dosing will lead to a steady state concentration in about seven weeks. 7
SULPHURis a naturally occurring mineral that is an essential part of the human body. It exhibits anti-bacterial, anti-parasitic, fungicidal, and keratolytic properties. 26Each tablet contains approximately 1.5 mg of sulphur (calculated). Sulphur is highly water soluble and as a result is easily excreted by the body via sweat and urine. 22Since the sulphur found in ECZEMOL ®is a naturally occurring mineral, it is radically different from sulfa drugs (sulfonamide antibiotics). Therefore, patients who are allergic to sulfa drugs CAN safely take ECZEMOL ®.
NICKEL SULPHATEdissolves and dissociates in the digestive tract into its ionic constituents. Each tablet contains approximately 0.5 mg of ionic nickel (calculated). According to studies, 15% to 50% of ionic nickel is absorbed on a fasted stomach. 2Food markedly decreases the rate and extent of nickel absorption. 3,4Clinical studies show that serum concentrations of nickel are variable among patients after administering the same dosage. 5Peak serum nickel concentration is reached about two hours after oral administration. "Once a day" dosing leads to steady state serum concentrations in approximately one week. Nickel is in its highly stable divalent cation state and is therefore not expected to be metabolized to any significant degree in the body. Absorbed nickel is primarily excreted in the urine and elimination half-life is about 21 hours. 3,5Renal clearance is rapid and efficient, and nickel does not accumulate in the body. 6
- CLINICAL STUDIES
- INDICATIONS
- CONTRAINDICATIONS
-
WARNING
Do not use if imprinted seal under bottle cap is missing or broken. Do not use if pregnant or nursing. If allergic to nickel or metal objects such as jewelry or if there is a history of blistering hand eczema, see PRECAUTIONSfor hypersensitivity information. Lactose intolerant patients may have gastrointestinal difficulty. This has very rarely been reported at the doses used.
-
PRECAUTIONS
Carefully adjust dosage to weight when treating young children. Use cautiously in setting of kidney disease. (see Dosage and Administration) If skin rash appears or if nervous symptoms persist, recur frequently, or are unusual, discontinue use.
Hypersensitivity
Caution should be used when administering to patients with a history of contact sensitivity to nickel (common metal exposure) or if there is a history of vesicular hand eczema (dyshidrosis, pomphylox). Nickel allergy may be confirmed by a positive nickel patch test. Most patients with hand eczema, positive nickel allergy history, or a positive nickel patch test do nothave any untoward reaction to administration of ECZEMOL ®. If there is a history of nickel sensitivity or dyshidrotic hand eczema, begin with a very low dose and slowly increase to a recommended starting dose over a period of 5 weeks as tolerated, thus allowing progressive GI absorption *.
*Nickel desensitization schedule: Week Amount of Time to Take
Medication Prior to BreakfastWeek 1 With Breakfast Week 2 15 min Prior Week 3 30 min Prior Week 4 45 min Prior Week 5 and thereon 1 hour Prior If new pruritic rashes occur or persist, discontinue ECZEMOL ®and treat appropriately. Do not use if there is a history of extra-cutaneous hypersensitivity to nickel or any ingredient in ECZEMOL ®.
Information for patients
Patients using ECZEMOL ®should receive the following information and instructions:
- This medication is to be used as directed by a physician.
- It is important to take orally at the beginning of the day on an empty stomach (or any convenient time after having taken nothing but water for at least 7 hours) and to eat or drink nothing but water for at least one hour afterwards to avoid interference with absorption.
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been done on the carcinogenesis, mutagenesis, or impairment of fertility of ECZEMOL ®. No carcinogenesis or mutagenesis has been reported in multiple animal studies for oral administration of soluble nickel and bromide salts (active ingredients) even at very high doses. 10-14
Effects of soluble potassium bromide
KBr is not listed as a carcinogen by the NTP, IARC, and OSHA. 16
Effects of sulphur
Sulphur is not listed as a carcinogen by the ACGIH, IARC, NIOSH, NTP, or OSHA. 25
Effects of soluble nickel sulphate
Studies on experimental animals have never indicated that nickel, at any dose, is a carcinogen when introduced to the body orally. Furthermore, Nickel sulphate and other highly water soluble nickel salts, have never been known to induce carcinogenesis via any route of introduction including: oral, inhalation, cutaneous, IM, or IP. 10-12,15No adverse effects were noted on fertility or reproduction in a 3-generational study of albino Wistar rats fed up to 1000 ppm Ni per day, which is equivalent to 50 mg/kg body weight per day Ni. 15
-
ADVERSE REACTIONS
ECZEMOL ®contains low doses of active ingredients. Therefore there are minimal known side effects. (see PRECAUTIONSfor hypersensitivity information)
-
OVERDOSAGE
Potassium bromide toxicity
Indications of toxicity due to oral overdosage of bromide may include nausea, vomiting, apathy, disturbed coordination, loss of memory, drowsiness, loss of emotional control, agitation, hallucination, tremors, depressed reflexes, stupor, and coma. Acute toxic reactions in humans have been reported at doses as low as 1000 mg. 19This level is 67 times the dose received in one tablet of ECZEMOL ®.
Sulphur toxicity
The oral rat LD 50for sulphur is reported to be greater than 5,000 mg/kg . 23This is more than 37,000 times the maximum dose recommended for ECZEMOL ®. (see Dosage) Ingestion of toxic levels of sulphur can cause sore throat, nausea, headache, gastrointestinal irritation, and possibly unconsciousness in severe cases. 24,25Sulphur poses such a remote risk that it is placed in the lowest toxic category possible, EPA Toxicity Category IV. 23
Nickel sulphate toxicity
The oral rat LD 50for nickel sulphate hexahydrate is 275 mg/kg. 17Symptoms of toxicity due to oral overdosage of nickel sulphate may include nausea, vomiting, abdominal discomfort, diarrhea, giddiness, lassitude, headaches, cough, and shortness of breath. 18The lowest observed transitory toxic effects from human ingestion of soluble nickel salts is approximately 8 mg nickel/kg body weight. 18This is 180 times the maximum dose recommended for ECZEMOL ®. (See below)
-
DOSAGE AND ADMINISTRATION
Absorption of nickel sulphate is variable among individuals. For maximum absorption, tablets should be taken orally at the beginning of the day(or any convenient time after having taken nothing but water for at least 7 hours). Take nothing but water for one hour after taking medication to aid absorption.
Kg lbs Starting
doseMax Daily dose 5-11 11-25 ¼ ½ 12-22 26-50 ½ 1 23-45 51-100 1 2 46-68 101-150 2 4 69-90 151-200 3 6 91+ 201+ 4 8 In the setting of renal impairment
Dosage should be adjusted and serum nickel and bromide levels should be followed. Steady state trough level should be drawn prior to ingesting the day's dose after one week of dosing or at appropriate intervals. Target trough serum nickel level is 20-40 mcg/L. (Caution: post dose peak levels are unreliable.) Treatment duration depends on the individual. Increase dose as needed on a monthly basis. Try b.i.d. dosing (upon rising and at bedtime) if max dose (see above) is not effective; do not exceed max daily dose.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ECZEMOL
potassium bromide, nickel sulfate, and sulfur tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61480-127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM BROMIDE (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) POTASSIUM BROMIDE 1 [hp_X] NICKEL SULFATE (UNII: 4FLT4T3WUN) (NICKEL CATION - UNII:OIS2CXW7AM) NICKEL SULFATE 1 [hp_X] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 1 [hp_X] Product Characteristics Color white (Off-White with Green Speckles) Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code LL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61480-127-05 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/15/2001 Labeler - PLYMOUTH HEALTHCARE PRODUCTS LLC (079330314)