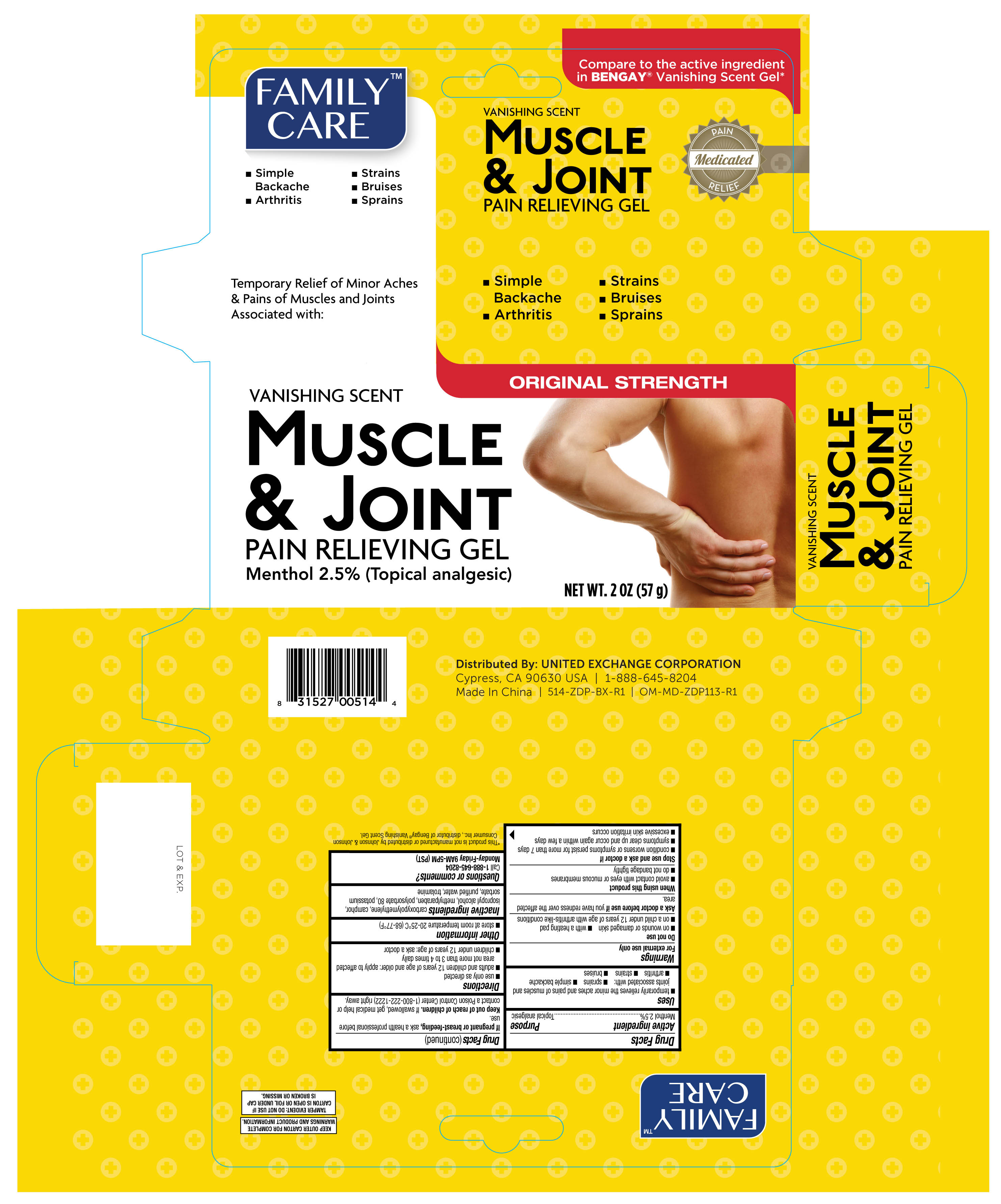
Label: FAMILY CARE MUSCLE AND JOINT- menthol gel
- NDC Code(s): 65923-144-57
- Packager: United Exchange Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FAMILY CARE MUSCLE AND JOINT
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65923-144 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 25 mg in 1 g Inactive Ingredients Ingredient Name Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) ISOPROPYL ALCOHOL (UNII: ND2M416302) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65923-144-57 1 in 1 CARTON 10/23/2019 1 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/23/2019 Labeler - United Exchange Corp. (840130579)