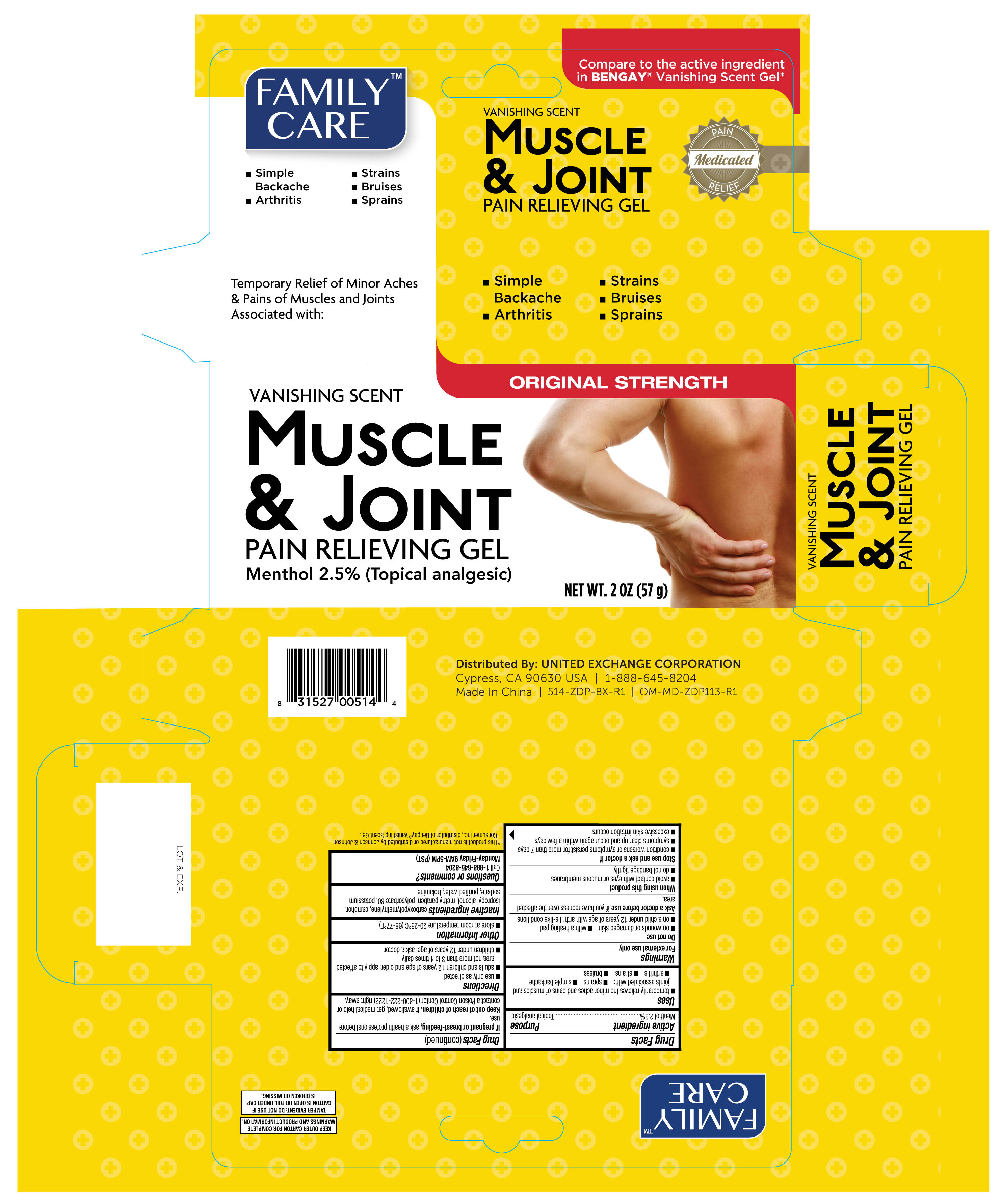
Active ingredient Purpose
Menthol 2.5%.................................................................Topical analgesic
Uses
- temporarily relieves the minor aches and pains of muscles and joints associated with:
- sprains
- simple backache
- arthritis
- strains
- bruises
Do not use
- on wounds or damaged skin
- with a heating pad
- on a child under 12 years of age with arthritis-like conditions
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive skin irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- use only as directed
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor