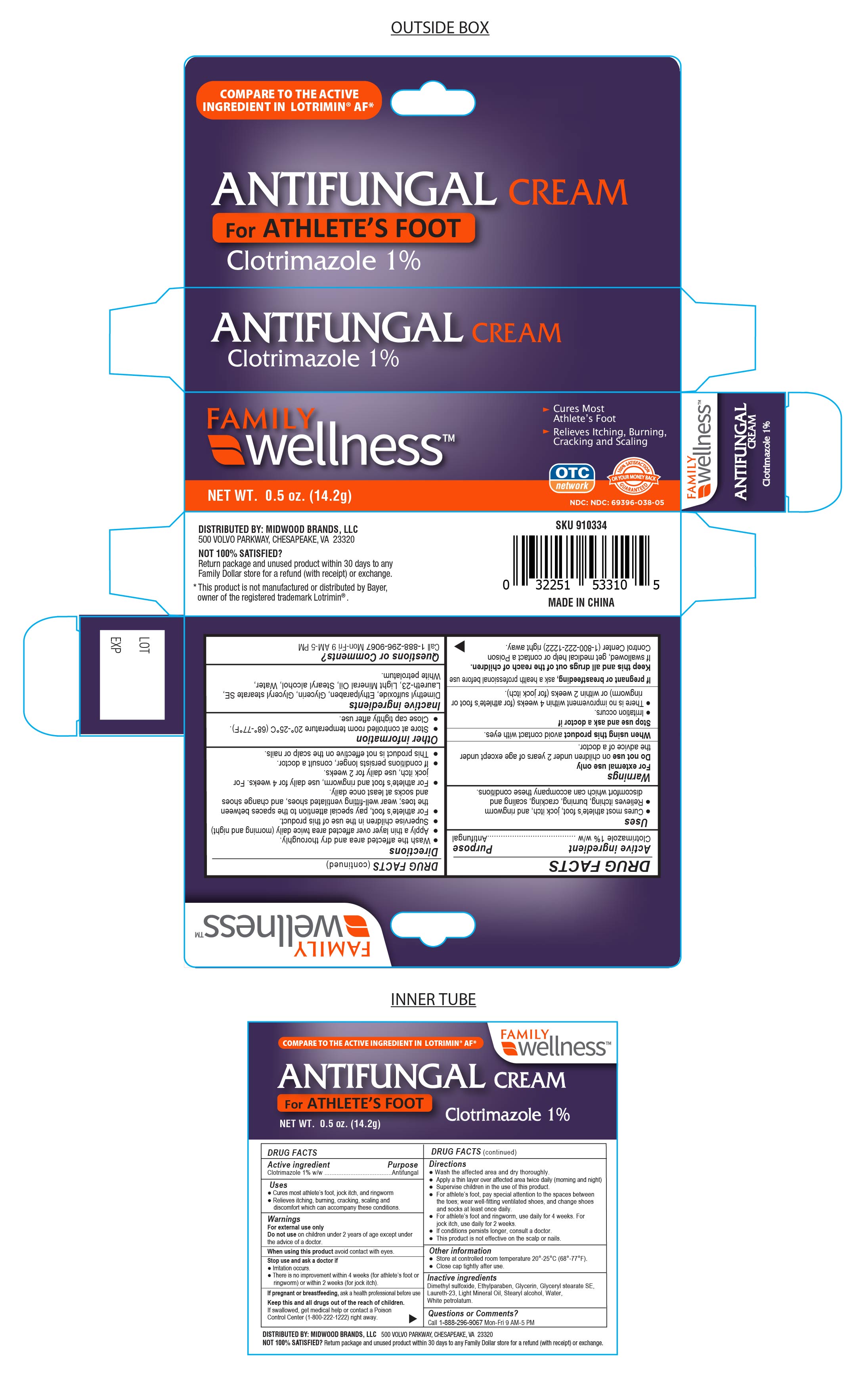
Label: CLOTRIMAZOLE- family wellness clotrimazole cream
- NDC Code(s): 69396-038-05
- Packager: Trifecta Pharmaceuticals USA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Uses
- Warnings
- Stop Use and ask a Doctor if:
- If Pregnant or breastfeeding
-
Directions
- Wash the affected area and dry thoroughly.
● Apply a thin layer of this product over affected area twice daily (morning and night), or as directed by a doctor.
● Supervise children in the use of this product.
● For athlete’s foot, pay special attention to the spaces between the toes; wear well-fitting ventilated shoes, and change shoes and socks at least once daily.
● For athlete’s foot and ringworm, use daily for 4 weeks. For jock itch, use daily for 2 weeks.
● If conditions persists longer, consult a doctor.
● This product is not effective on the scalp or nails.
- Inactive Ingredients
- Other Information
-
Questions or Comments?
Call 1-888-296-9067 Mon-Fri 9AM - 5PM
DISTRIBUTED BY: MIDWOOD BRANDS, LLC.
500 Volvo Parkway, Chesapeake, VA. 23320
NOT SATISFIED?
Return Package and unused product within 30 days to any Family Dollar store for a refund (with receipt) or exchange.
This product is not manufactured or distributed by Bayer, owner of the registered trademark Lotrimin®
- Packaging
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE
family wellness clotrimazole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69396-038 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ETHYLPARABEN (UNII: 14255EXE39) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) LAURETH-23 (UNII: N72LMW566G) LIGHT MINERAL OIL (UNII: N6K5787QVP) PETROLATUM (UNII: 4T6H12BN9U) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69396-038-05 1 in 1 BOX 08/30/2019 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 08/30/2019 Labeler - Trifecta Pharmaceuticals USA LLC (079424163)