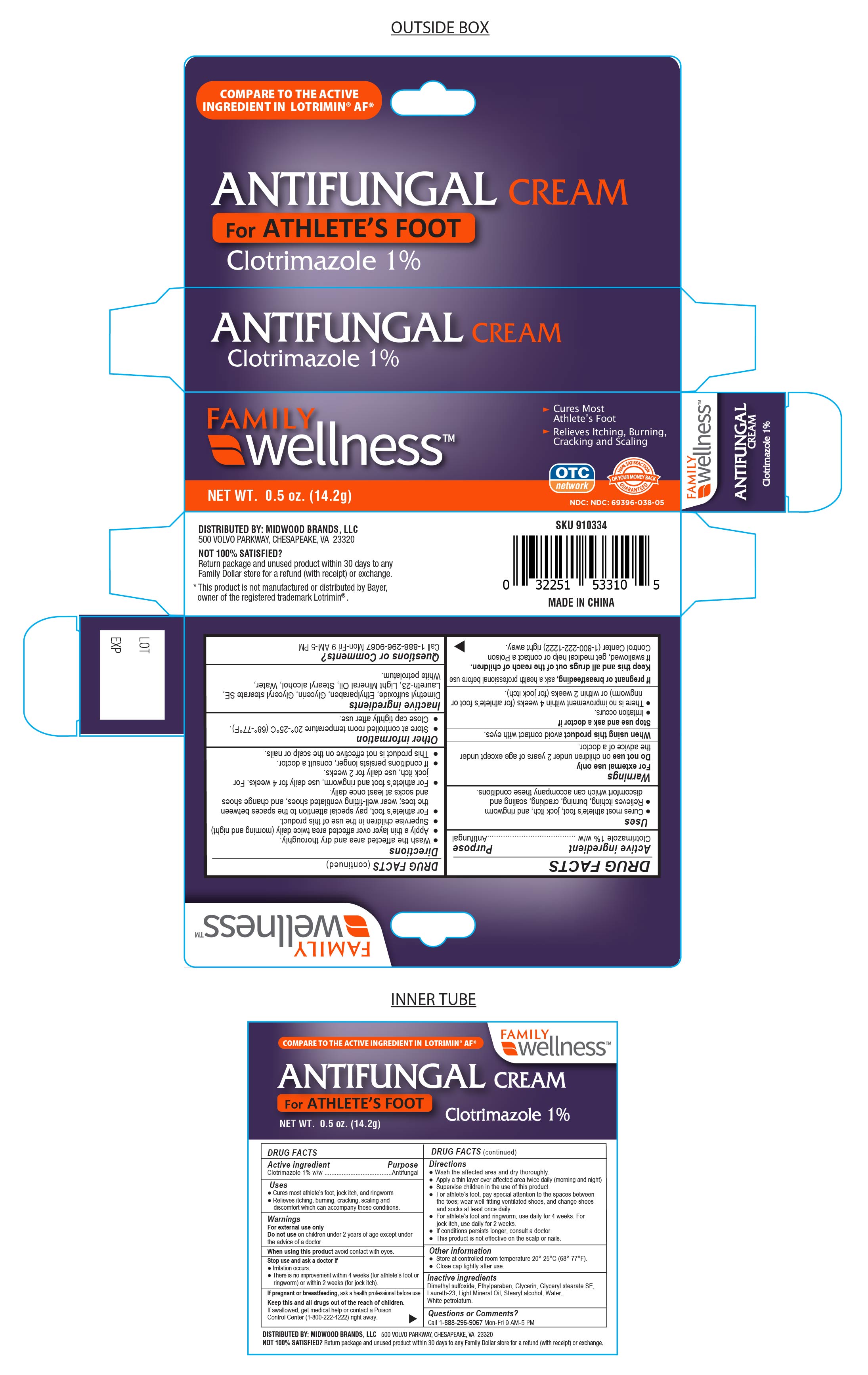
Keep this and all drugs out of the reach of children. If swallowed get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Uses
Cures athlete’s foot (tinea pedis), jock itch (tinea cruris), ringworm (tinea corporis). Relieves the itching, burning, cracking, scaling and discomfort which can accompany these conditions.
Warnings
● Do not use on children under 2 years of age except under the advice of a doctor.
● For external use only
● When using this product avoid contact with eyes.
Stop Use and ask a Doctor if:
- irritation occurs
- there is no improvement within 4 weeks (for Athlete's foot or ringwork) or within 2 weeks (for jock itch).
Directions
- Wash the affected area and dry thoroughly.
● Apply a thin layer of this product over affected area twice daily (morning and night), or as directed by a doctor.
● Supervise children in the use of this product.
● For athlete’s foot, pay special attention to the spaces between the toes; wear well-fitting ventilated shoes, and change shoes and socks at least once daily.
● For athlete’s foot and ringworm, use daily for 4 weeks. For jock itch, use daily for 2 weeks.
● If conditions persists longer, consult a doctor.
● This product is not effective on the scalp or nails.
Inactive Ingredients
Dimethyl Sulfoxide, Ethylparaben, Glycerin, Glyceryl Stearate SE, Laureth-23, Light Mineral Oil, Petrolatum, Stearyl Alcohol, Water
Other Information
- store at controlled room temperature 20°-25°C (68°- 77°F)
- Close cap tightly after use.
Questions or Comments?
Call 1-888-296-9067 Mon-Fri 9AM - 5PM
DISTRIBUTED BY: MIDWOOD BRANDS, LLC.
500 Volvo Parkway, Chesapeake, VA. 23320
NOT SATISFIED?
Return Package and unused product within 30 days to any Family Dollar store for a refund (with receipt) or exchange.
This product is not manufactured or distributed by Bayer, owner of the registered trademark Lotrimin®