Label: THERATEARS LUBRICANT- carboxymethylcellulose sodium solution/ drops
-
NDC Code(s):
58790-000-00,
58790-000-20,
58790-000-24,
58790-000-30, view more58790-000-32
- Packager: MEDTECH PRODUCTS INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
THERATEARS LUBRICANT- carboxymethylcellulose sodium solution/ drops
Medtech Products Inc., a Prestige Consumer Healthcare companyDisclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
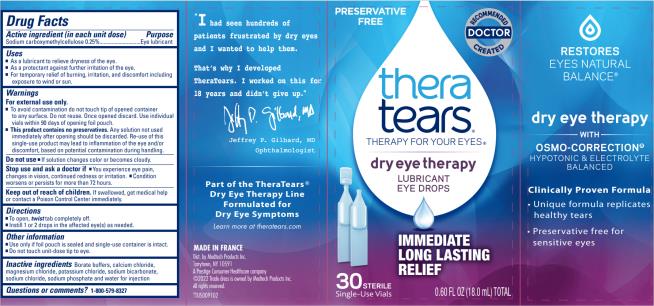
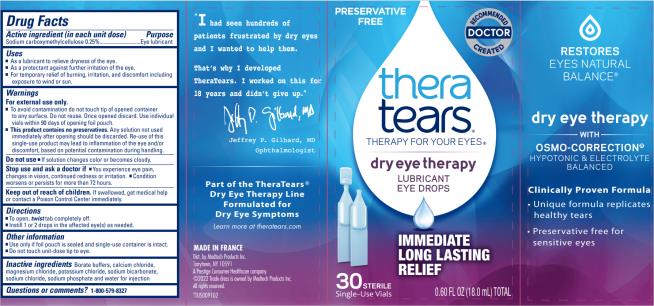
Drug Facts
- Active ingredient (In each unit dose)
- Purpose
- Uses
-
Warnings
For external use only
- To avoid contamination do not touch tip of opened container to any surface. Do not reuse. Once opened discard. Use individual vials within 90 days of opening foil pouch.
- This product contains no preservatives. Any solution not used immediately after opening should be discarded. Re-use of this single-use product may lead to inflammation of the eye and/or discomfort, based on ptential contamination during handling.
- To avoid contamination do not touch tip of opened container to any surface. Do not reuse. Once opened discard. Use individual vials within 90 days of opening foil pouch.
- Directions
- Other information
- Inactive ingredients
- Questions and comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
THERATEARS LUBRICANT
carboxymethylcellulose sodium solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58790-000 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength carboxymethylcellulose sodium, unspecified form (UNII: K679OBS311) (carboxymethylcellulose - UNII:05JZI7B19X) carboxymethylcellulose sodium, unspecified form 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength boric acid (UNII: R57ZHV85D4) sodium borate (UNII: 91MBZ8H3QO) calcium chloride (UNII: M4I0D6VV5M) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) water (UNII: 059QF0KO0R) sodium bicarbonate (UNII: 8MDF5V39QO) sodium chloride (UNII: 451W47IQ8X) sodium phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58790-000-32 8 in 1 CARTON 01/01/1995 1 4 in 1 POUCH 1 19.2 mL in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:58790-000-24 6 in 1 CARTON 01/01/1995 2 4 in 1 POUCH 2 19.2 mL in 1 APPLICATOR; Type 0: Not a Combination Product 3 NDC:58790-000-20 5 in 1 CARTON 01/01/1995 3 4 in 1 POUCH 3 19.2 mL in 1 APPLICATOR; Type 0: Not a Combination Product 4 NDC:58790-000-30 6 in 1 CARTON 12/07/2018 4 5 in 1 POUCH 4 0.6 mL in 1 VIAL; Type 0: Not a Combination Product 5 NDC:58790-000-00 6 in 1 CARTON 12/07/2018 5 5 in 1 POUCH 5 0.6 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 01/01/1995 Labeler - MEDTECH PRODUCTS INC (114707784)