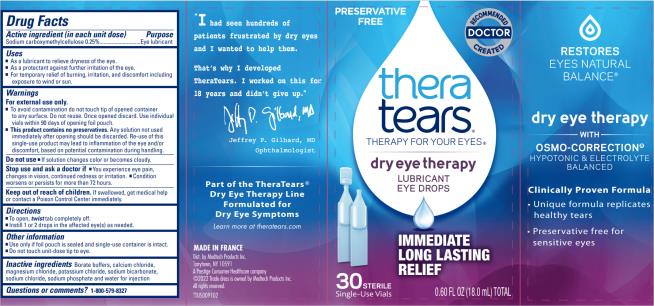
THERATEARS LUBRICANT- carboxymethylcellulose sodium solution/ drops
Medtech Products Inc., a Prestige Consumer Healthcare company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- As a lubricant to relieve dryness of the eye.
- As a protectant against further irritation of the eye.
- For temporary relief of burning, irritation, and discomfort including exposure to wind or sun.
Warnings
For external use only
- To avoid contamination do not touch tip of opened container to any surface. Do not reuse. Once opened discard. Use individual vials within 90 days of opening foil pouch.
- This product contains no preservatives. Any solution not used immediately after opening should be discarded. Re-use of this single-use product may lead to inflammation of the eye and/or discomfort, based on ptential contamination during handling.
Directions
- To open, twist tab completely off.
- Instill 1 or 2 drops in the affected eye(s) as needed.
Other information
- Use only if foil pouch is sealed and single-use container is intact.
- Do not touch unit-dose tip to eye.