Label: KRYSTEXXA- pegloticase injection, solution
- NDC Code(s): 75987-080-10
- Packager: Horizon Therapeutics USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KRYSTEXXA safely and effectively. See full prescribing information for KRYSTEXXA - KRYSTEXXA® (pegloticase) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ANAPHYLAXIS and INFUSION REACTIONS, G6PD DEFICIENCY ASSOCIATED HEMOLYSIS and METHEMOGLOBINEMIA
- Anaphylaxis and infusion reactions have been reported to occur during and after administration of KRYSTEXXA. (5.1, 5.2)
- Anaphylaxis may occur with any infusion, and generally manifests within 2 hours of the infusion. However, delayed hypersensitivity reactions have also been reported. (5.1)
- KRYSTEXXA should be administered in healthcare settings and by healthcare providers prepared to manage anaphylaxis and infusion reactions. (5.1, 5.2)
- Premedicate with antihistamines and corticosteroids and closely monitor for anaphylaxis for an appropriate period of time after administration of KRYSTEXXA. (5.1, 5.2)
- Monitor serum uric acid levels prior to each infusion and discontinue treatment if levels increase to above 6 mg/dL, particularly when 2 consecutive levels above 6 mg/dL are observed. (5.2)
- Screen patients at risk for G6PD deficiency prior to starting KRYSTEXXA. Hemolysis and methemoglobinemia have been reported with KRYSTEXXA in patients with G6PD deficiency. KRYSTEXXA is contraindicated in patients with G6PD deficiency. (4, 5.3)
-
1 INDICATIONS AND USAGEKRYSTEXXA® (pegloticase) is indicated, for the treatment of chronic gout in adult patients refractory to conventional therapy. Gout refractory to conventional therapy occurs in patients who have ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Precautions Prior to Treatment - It is recommended that before starting KRYSTEXXA patients discontinue oral urate-lowering medications and not ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 8 mg/mL as a clear and colorless solution for dilution in a single-dose vial. KRYSTEXXA concentrations are expressed as concentrations of uricase protein. Each mL of pegloticase ...
-
4 CONTRAINDICATIONSKRYSTEXXA is contraindicated in: Patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency [see Warnings and Precautions (5.3)] Patients with history of serious hypersensitivity ...
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylaxis - In a 52-week controlled trial, which evaluated KRYSTEXXA co-administered with methotrexate compared to KRYSTEXXA alone, patients were pre-treated with standardized infusion ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of the label: Anaphylaxis [see Warnings and Precautions (5.1)] Infusion Reactions [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Methotrexate - KRYSTEXXA 8 mg every 2 weeks has been studied in patients with chronic gout refractory to conventional therapy taking concomitant oral methotrexate 15 mg weekly [see Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of KRYSTEXXA in pregnant women. Based on animal reproduction studies, no structural abnormalities were observed ...
-
10 OVERDOSAGENo reports of overdosage with KRYSTEXXA have been reported. The maximum dose that has been administered as a single intravenous dose is 12 mg as uricase protein. Patients suspected of receiving an ...
-
11 DESCRIPTIONKRYSTEXXA (pegloticase) is a uric acid specific enzyme which is a PEGylated product that consists of recombinant modified mammalian urate oxidase (uricase) produced by a genetically modified ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - KRYSTEXXA is a uric acid specific enzyme which is a recombinant uricase and achieves its therapeutic effect by catalyzing the oxidation of uric acid to allantoin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of pegloticase. The genotoxic potential of ...
-
14 CLINICAL STUDIESCo-administration with Methotrexate - A 52-week, randomized, double-blind trial was conducted in adult patients with chronic gout refractory to conventional therapy, to evaluate administration ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - KRYSTEXXA (pegloticase) injection is supplied as a sterile, clear and colorless solution containing 8 mg of uricase protein in 1 mL in phosphate buffered saline intended for ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Anaphylaxis and Infusion Reactions - Anaphylaxis and infusion reactions can occur at any infusion while on ...
-
SPL UNCLASSIFIED SECTIONAMGEN - Manufactured by: Horizon Therapeutics Ireland DAC - Dublin, Ireland - US License Number 2022 - Patent: https://pat.amgen.com/krystexxa/ ©2025 Horizon Therapeutics Ireland DAC. All rights ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 01/2025 - Medication Guide - KRYSTEXXA® (kris-TEX-a) (pegloticase) injection, for ...
-
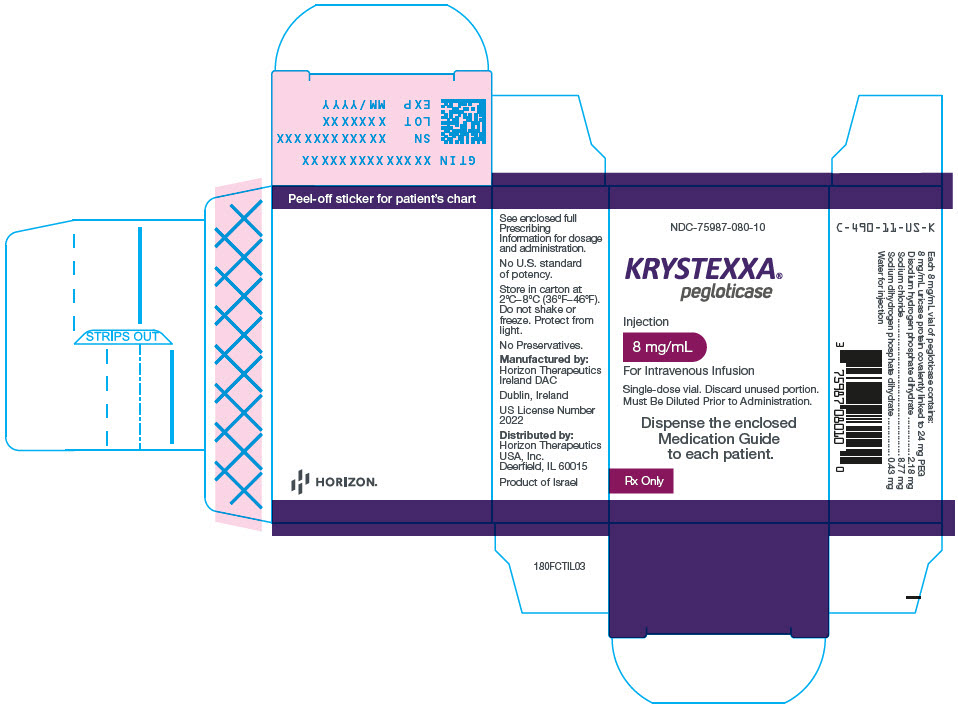
PRINCIPAL DISPLAY PANEL - 8 mg/mL Vial CartonNDC-75987-080-10 - KRYSTEXXA® pegloticase - Injection - 8 mg/mL - For Intravenous Infusion - Single-dose vial. Discard unused portion. Must Be Diluted Prior to Administration. Dispense the enclosed ...
-
INGREDIENTS AND APPEARANCEProduct Information