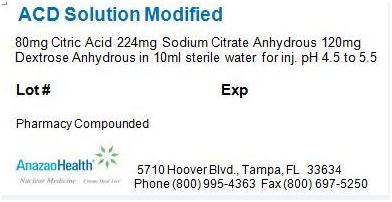
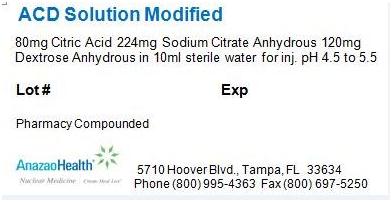
Label: ACD SOLUTION MODIFIED solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 51808-201-01 - Packager: AnazaoHealth Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 23, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
In vitro, citrate ions combine with ionic calcium in the blood and the resulting
lack of ionic calcium prevents coagulation. Blood that has been treated with citrate anticoagulants is nontoxic to the body when injected in small amounts intravenously. After injection, citrate ions are rapidly removed from the blood by the liver, polymerized into glucose, and then metabolized in the usual manner
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
DOSAGE AND ADMINISTRATION
Red Blood Cell Labeling Procedure
- Labeling may be performed without washing or centrifugation steps directly in the reaction vial.
- A 30 to 50 mL sample of whole blood is withdrawn from the patient and added aseptically to a vial of ACD Solution Modified.
- 50 to 150 microcuries of Sodium Chromate 51 is then injected into the reaction vial using a shielded syringe. The amount of radioactivity added to the vial will
depend on the intended use of the labeled red blood cells.
- The suspension is incubated for 30 to 60 minutes at room temperature with frequent, gentle agitation.
- After incubation, 100 mg Ascorbic Acid Injection is injected into the vial. The ascorbic acid reduces any remaining unbound dianionic chromium 51 to the anionic state which does not penetrate red blood cells; thus in vivo labeling of red blood cells is prevented.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACD SOLUTION MODIFIED
acd solution modified solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51808-201 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 8 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) 22.4 mg in 1 mL ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) 12 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51808-201-01 10 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 05/23/2012 Labeler - AnazaoHealth Corporation (011038762) Establishment Name Address ID/FEI Business Operations AnazaoHealth Corporation 011038762 MANUFACTURE