Per your order, we have compounded ACD Solution Modified as a solution of 10 mL in a 100 mL vial. The characteristics of this compounded preparation are as follows
DESCRIPTION
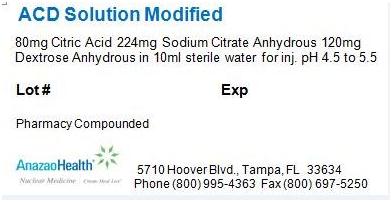
Each 100 mL vial contains 80 mg citric acid, 224 mg sodium citrate anhydrous, and 120 mg dextrose anhydrous in a sterile, non-pyrogenic solution of 10 mL. The pH of the solution has been adjusted to be between 4.5 to 5.5
CLINICAL PHARMACOLOGY
In vitro, citrate ions combine with ionic calcium in the blood and the resulting
lack of ionic calcium prevents coagulation. Blood that has been treated with citrate anticoagulants is nontoxic to the body when injected in small amounts intravenously. After injection, citrate ions are rapidly removed from the blood by the liver, polymerized into glucose, and then metabolized in the usual manner
INDICATIONS AND USAGE
ACD solution modified is to be used in the labeling of red blood cells for intravenous administration with Cr-51 Sodium Chromate.
DOSAGE AND ADMINISTRATION
Red Blood Cell Labeling Procedure
- Labeling may be performed without washing or centrifugation steps directly in the reaction vial.
- A 30 to 50 mL sample of whole blood is withdrawn from the patient and added aseptically to a vial of ACD Solution Modified.
- 50 to 150 microcuries of Sodium Chromate 51 is then injected into the reaction vial using a shielded syringe. The amount of radioactivity added to the vial will
depend on the intended use of the labeled red blood cells.
- The suspension is incubated for 30 to 60 minutes at room temperature with frequent, gentle agitation.
- After incubation, 100 mg Ascorbic Acid Injection is injected into the vial. The ascorbic acid reduces any remaining unbound dianionic chromium 51 to the anionic state which does not penetrate red blood cells; thus in vivo labeling of red blood cells is prevented.