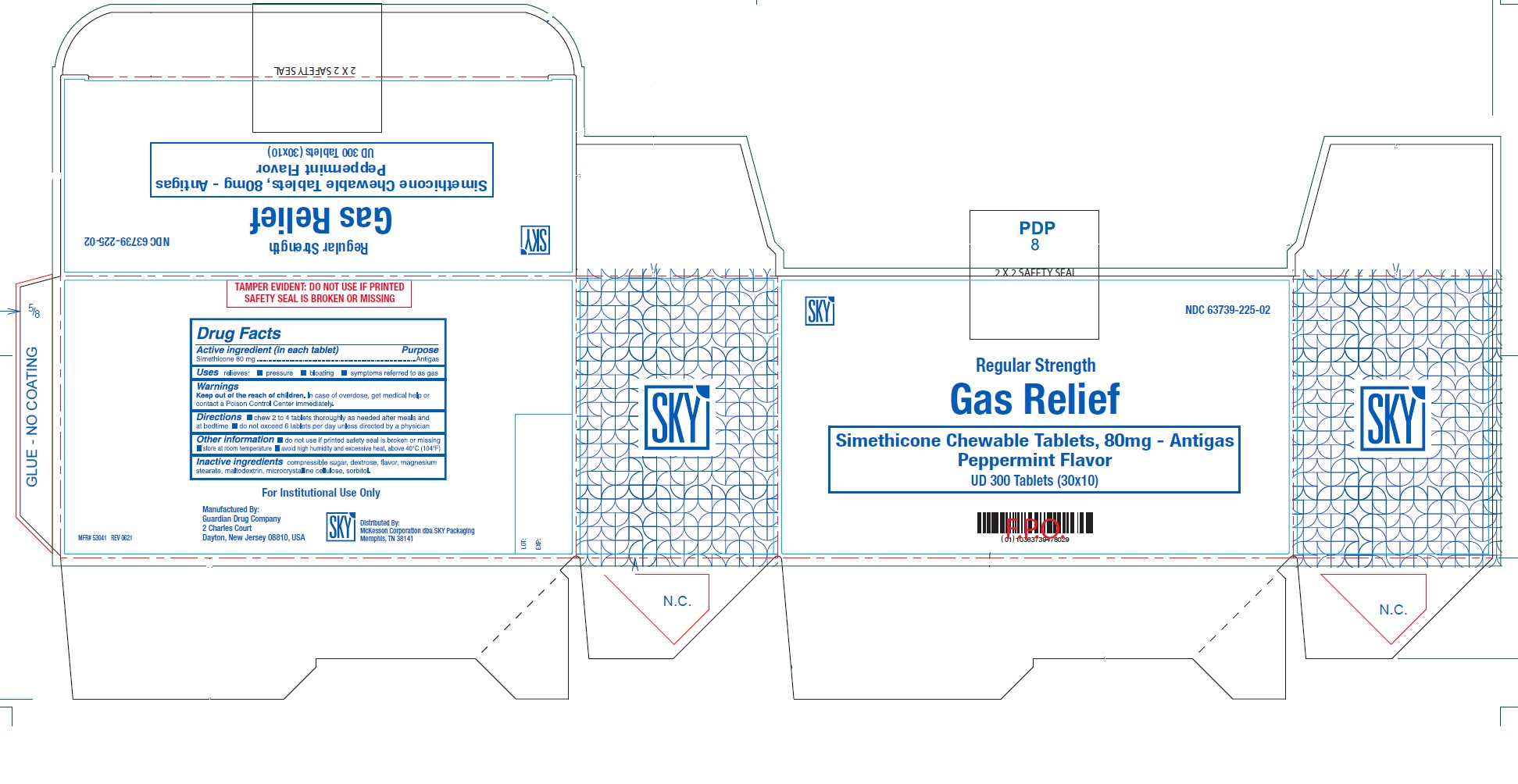
Label: GAS RELIEF- simethicone tablet, chewable
- NDC Code(s): 63739-225-02
- Packager: McKesson Corporation dba SKY Packaging
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(in each tablet)
- PURPOSE
- USE(S)
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GAS RELIEF
simethicone tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63739-225 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 80 mg Inactive Ingredients Ingredient Name Strength SUGARCANE (UNII: 81H2R5AOH3) MALTODEXTRIN (UNII: 7CVR7L4A2D) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL (UNII: 506T60A25R) Product Characteristics Color WHITE Score no score Shape ROUND Size 13mm Flavor PEPPERMINT Imprint Code G103 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63739-225-02 30 in 1 BOX 10/19/2021 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 10/19/2021 Labeler - McKesson Corporation dba SKY Packaging (140529962) Establishment Name Address ID/FEI Business Operations Guardian Drug Company 119210276 MANUFACTURE(63739-225)