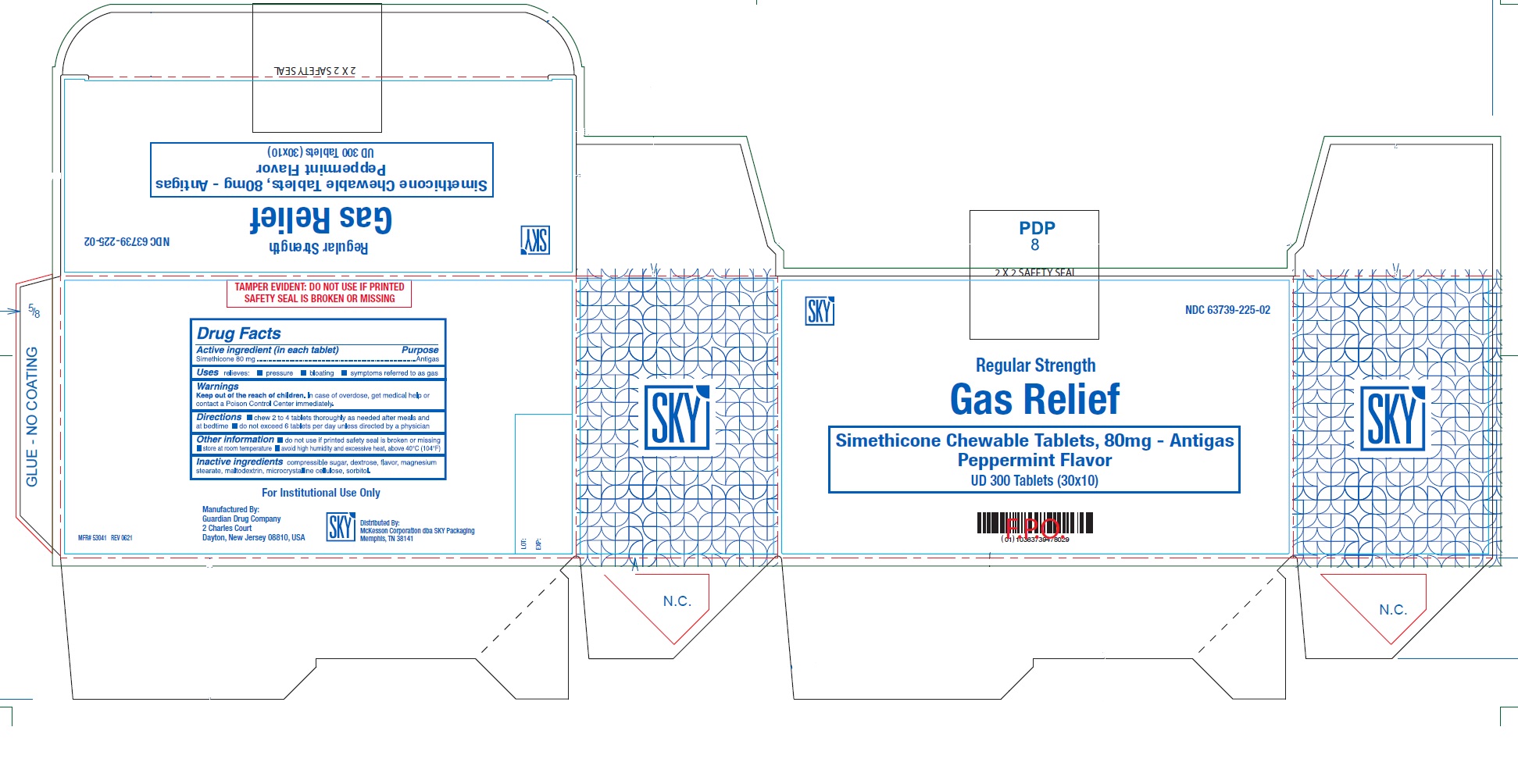
GAS RELIEF - simethicone tablet, chewable
McKesson Corporation dba SKY Packaging
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT(in each tablet)
Simethicone 80 mg
USE(S)
relieves:
- pressure
- bloating
- symptoms referred to as gas
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center immediately.
DIRECTIONS
- chew 2 to 4 tablets thoroughly as needed after meals and at bedtime
- do not exceed 6 tablets per day unless directed by a physician
OTHER INFORMATION
- do not use if inner seal is broken or missing
- store at room temperature
- avoid high humidity and excessive heat, above 40oC (104oF)
INACTIVE INGREDIENT
compressible sugar, dextrose, flavor, magnesium stearate, maltodextrin, microcrystalline cellulose, sorbitol.
PRINCIPAL DISPLAY PANEL
NDC 63739-225-02
SKY
Regular Strength
Gas Relief
Simethicone Chewable Tablets, 80 mg - Antigas
Peppermint Flavor
UD 300 Tablets (30x10)
For Institutional Use Only