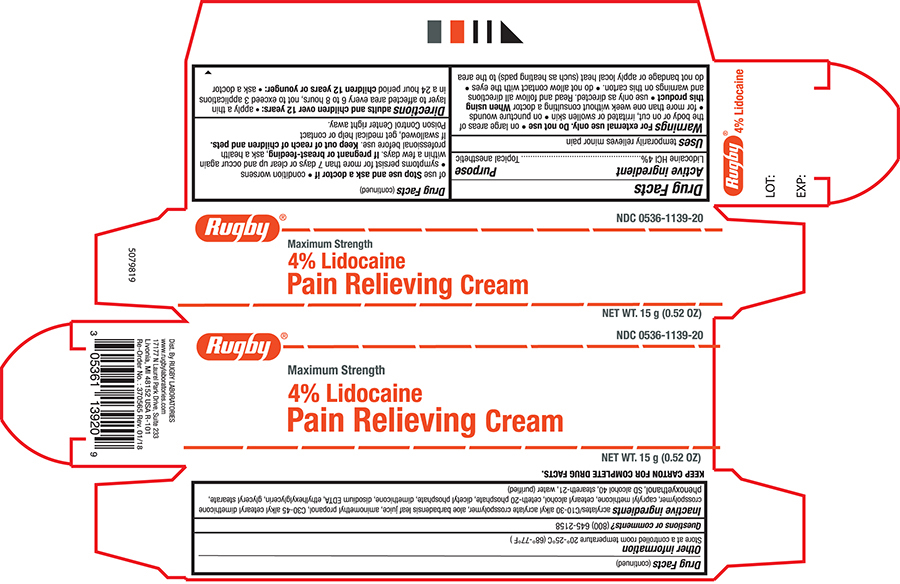
Label: RUGBY LIDOCAINE- lidocaine cream
- NDC Code(s): 0536-1139-20, 0536-1139-28
- Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- on large areas of the body or on cut,irritated or swollen skin
- on puncture wounds
- for more than one week without consultanting a doctor
- Directions
- Other information
-
Inactive ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Aminomethyl Propanol, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, Caprylyl Methicone, Cetearyl Alcohol, Ceteth-20 Phosphate, Dicetyl Phosphate, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glyceryl Stearate, Phenoxyethanol, SD Alcohol 40, Steareth-21, Water (purified)
- Questions?(800)645-2158
- Principal Panel-Tube
- Principal Panel - Carton
-
INGREDIENTS AND APPEARANCE
RUGBY LIDOCAINE
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1139 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 40 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SILICON (UNII: Z4152N8IUI) ALCOHOL (UNII: 3K9958V90M) CARBOMER INTERPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 132584PQMO) DIMETHICONE 350 (UNII: 2Y53S6ATLU) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARETH-21 (UNII: 53J3F32P58) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ALOE VERA LEAF (UNII: ZY81Z83H0X) CETEARETH-2 PHOSPHATE (UNII: 8NSU66JGZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1139-20 1 in 1 CARTON 09/21/2018 1 14 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0536-1139-28 1 in 1 CARTON 11/27/2018 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/21/2018 Labeler - Rugby Laboratories (079246066)