RUGBY LIDOCAINE- lidocaine cream
Rugby Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
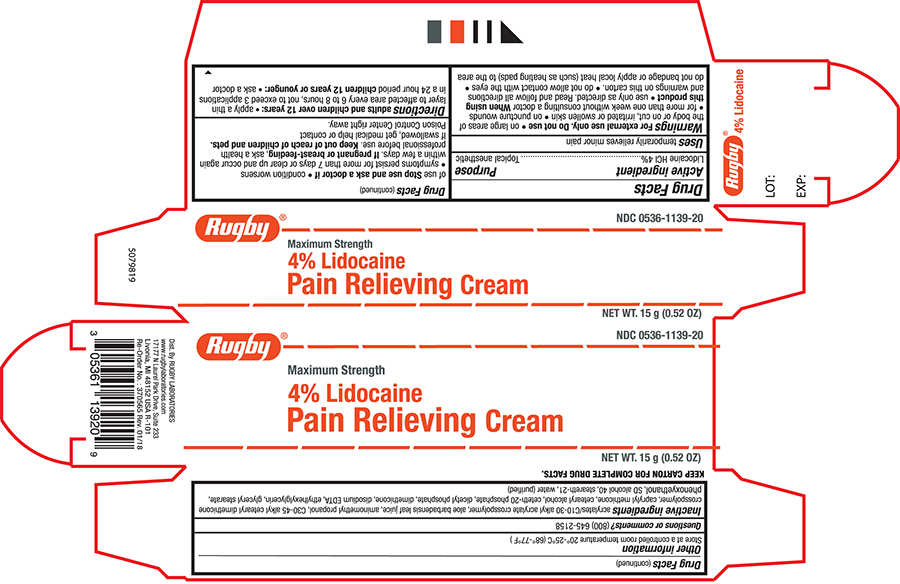
Lidocaine HCL 4%
Purpose
Topical anesthetic
Uses
- Temporarily relieves minor pain
Warnings
For external use only
Do not use
- on large areas of the body or on cut,irritated or swollen skin
- on puncture wounds
- for more than one week without consultanting a doctor
When using this product
use only as directed. read and follow all directions and warnings on this carton.
do notallow contact with the eyes
do not bandage or apply local heat (such as heating pads) to the area of use.
Stop use and ask a doctor if
- condition worsens
- symptons persist for more than 7 days or clear up and occur again within a few days.
-
If pregnant or breast feeding, ask a health professional before use.
Keep this and all drugs out of the reach of children and pets.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
-
Adults and children over 12 years:
- apply a thin layer ot affected area every 6 t o8 hours, not to exceed 3 applications in a 24 hour period
-
Children under 12 years or younger: ask a doctor
Other information
- Store at controlled room temperature 20°-25°C (68°-77°F)
Inactive ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Aminomethyl Propanol, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, Caprylyl Methicone, Cetearyl Alcohol, Ceteth-20 Phosphate, Dicetyl Phosphate, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glyceryl Stearate, Phenoxyethanol, SD Alcohol 40, Steareth-21, Water (purified)
Principal Panel-Tube
RUGBY® NDC 0536-1139-20
Maxium Strength
4% Lidocaine
Pain Relieving Cream
NET WT. 15g (0.52 oz)

Principal Panel - Carton
RUGBY® NDC 0536-1139-20
Maxium Strength
4% Lidocaine
Pain Relieving Cream
NET WT. 15g (0.52 oz)