Label: KWAN LOONG- methyl salicylate and menthol oil
- NDC Code(s): 66761-311-01, 66761-311-22
- Packager: Haw Par Healthcare Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
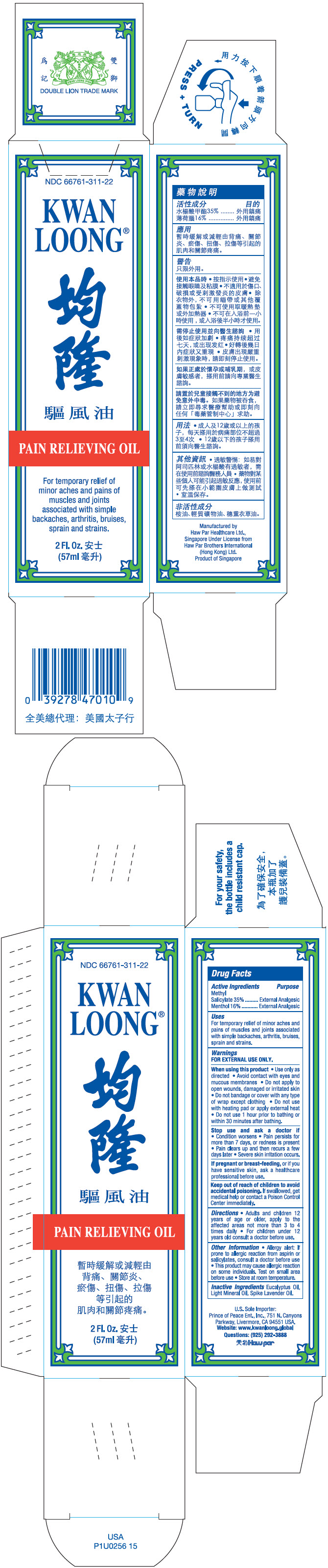
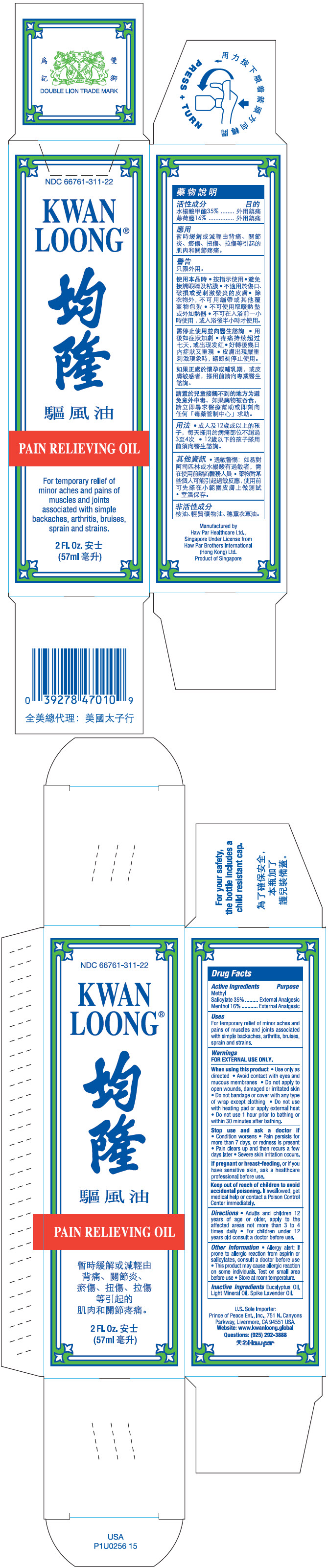
- ACTIVE INGREDIENT
- Uses
-
Warnings
FOR EXTERNAL USE ONLY.
When using this product
- Use only as directed
- Avoid contact with eyes and mucous membranes
- Do not apply to open wounds, damaged or irritated skin
- Do not bandage or cover with any type of wrap except clothing
- Do not use with heating pad or apply external heat
- Do not use 1 hour prior to bathing or within 30 minutes after bathing.
Stop use and ask a doctor if
- Condition worsens
- Pain persists for more than 7 days, or redness is present
- Pain clears up and then recurs a few days later
- Severe skin irritation occurs.
- Directions
- Other Information
- Inactive Ingredients
- Questions
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 57 ml Bottle Box
-
INGREDIENTS AND APPEARANCE
KWAN LOONG
methyl salicylate and menthol oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66761-311 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methyl salicylate (UNII: LAV5U5022Y) (Salicylic Acid - UNII:O414PZ4LPZ) Methyl salicylate 350 mg in 1 mL Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 160 mg in 1 mL Inactive Ingredients Ingredient Name Strength Eucalyptus Oil (UNII: 2R04ONI662) Spike Lavender Oil (UNII: 7S2HYV1VJQ) Light Mineral Oil (UNII: N6K5787QVP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66761-311-01 1 in 1 BOX 09/01/2005 1 28 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:66761-311-22 1 in 1 BOX 09/01/2005 2 57 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/01/2005 Labeler - Haw Par Healthcare Ltd. (659207039) Establishment Name Address ID/FEI Business Operations Haw Par Healthcare Ltd. 659207039 MANUFACTURE(66761-311) Establishment Name Address ID/FEI Business Operations Tiger Balm (Malaysia) Sdn. Bhd. 652104126 MANUFACTURE(66761-311) Establishment Name Address ID/FEI Business Operations Tiger Balm (Malaysia) Sdn. Bhd. 813058014 MANUFACTURE(66761-311)