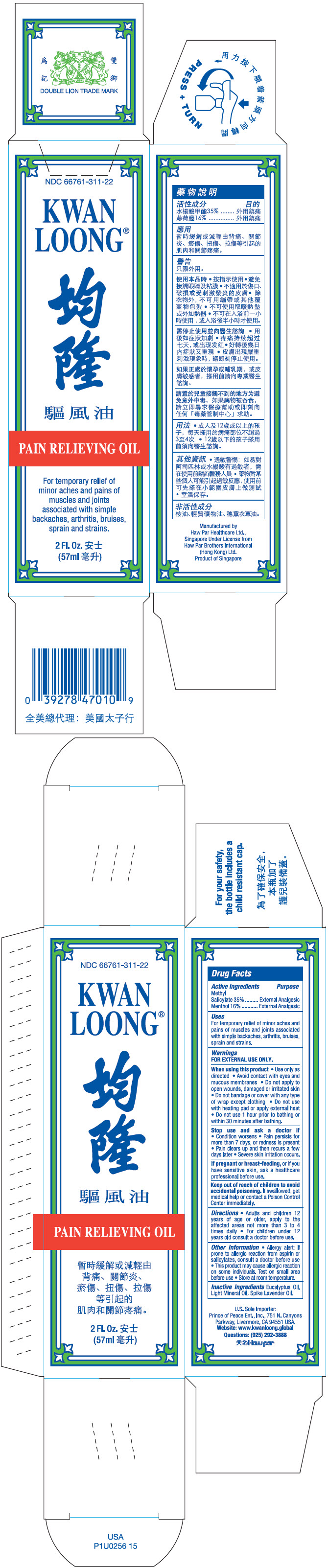
| Active Ingredients | Purpose |
| Methyl Salicylate 35% | External Analgesic |
| Menthol 16% | External Analgesic |
Uses
For temporary relief of minor aches and pains of muscles and joints associated with simple backaches, arthritis, bruises, sprain and strains.
Warnings
FOR EXTERNAL USE ONLY.
When using this product
- Use only as directed
- Avoid contact with eyes and mucous membranes
- Do not apply to open wounds, damaged or irritated skin
- Do not bandage or cover with any type of wrap except clothing
- Do not use with heating pad or apply external heat
- Do not use 1 hour prior to bathing or within 30 minutes after bathing.
Stop use and ask a doctor if
- Condition worsens
- Pain persists for more than 7 days, or redness is present
- Pain clears up and then recurs a few days later
- Severe skin irritation occurs.
If pregnant or breast-feeding, or if you have sensitive skin, ask a healthcare professional before use.
Keep out of reach of children to avoid accidental poisoning. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Adults and children 12 years of age or older, apply to the affected areas not more than 3 to 4 times daily
- For children under 12 years old consult a doctor before use.
Other Information
- Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use
- This product may cause allergic reaction on some individuals. Test on small area before use.
- Store at room temperature.
Inactive Ingredients
Eucalyptus Oil, Light Mineral Oil, Spike Lavender Oil.
Manufactured by
Haw Par Healthcare Ltd.,
Singapore
PRINCIPAL DISPLAY PANEL - 57 ml Bottle Box
NDC 66761-311-22
KWAN
LOONG®
PAIN RELIEVING OIL
For temporary relief of
minor aches and pains of
muscles and joints
associated with simple
backaches, arthritis, bruises,
sprain and strains.
2 Fl. Oz.
(57ml)
Haw Par Healthcare Ltd.