Label: ZEPHREX D- pseudoephedrine hydrochloride capsule, gelatin coated
- NDC Code(s): 0113-0401-62, 0113-0401-67
- Packager: L. Perrigo Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
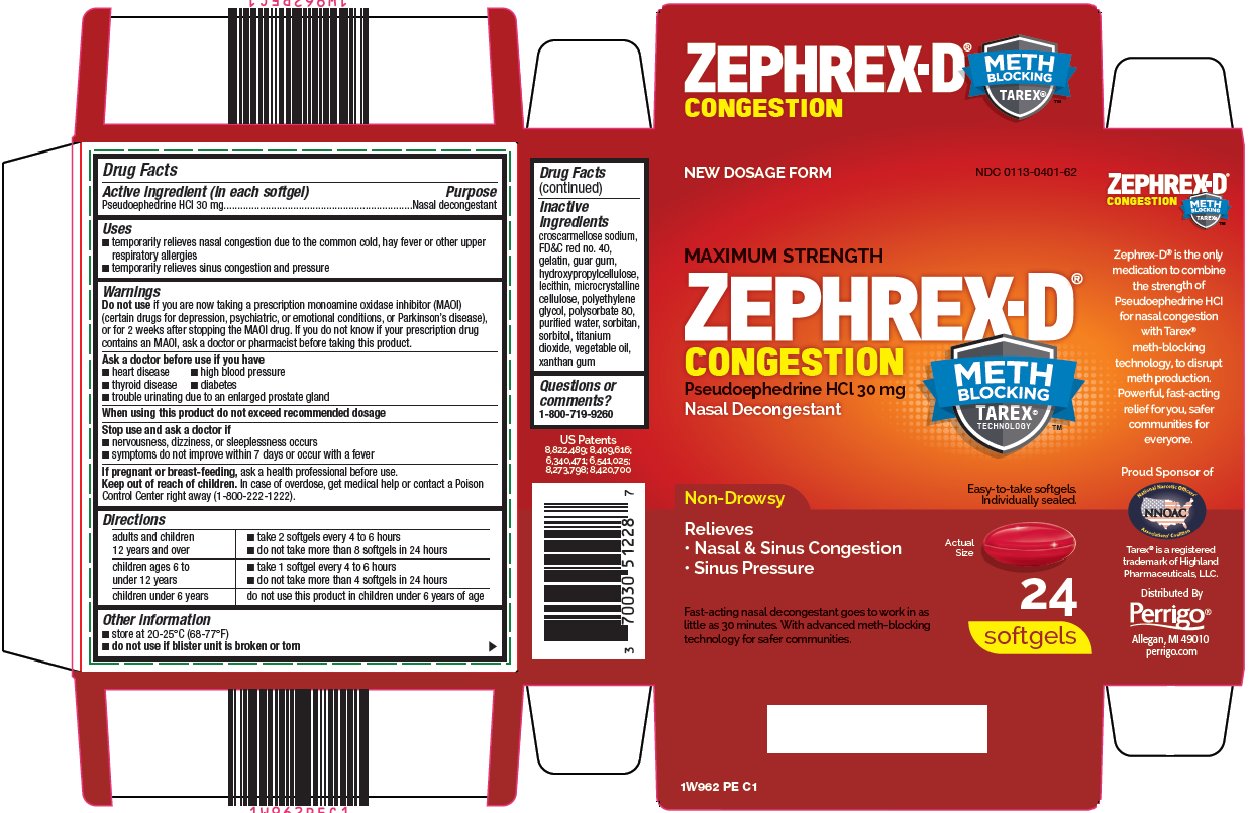
- Active ingredient (in each softgel)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
-
Directions
adults and children 12 years and over
- •
- take 2 softgels every 4 to 6 hours
- •
- do not take more than 8 softgels in 24 hours
children ages 6 to under 12 years
- •
- take 1 softgel every 4 to 6 hours
- •
- do not take more than 4 softgels in 24 hours
children under 6 years
do not use this product in children under 6 years of age
- Other information
- Inactive ingredients
- Questions or comments?
-
Package/Label Principal Display Panel
NEW DOSAGE FORM
MAXIMUM STRENGTH
ZEPHREX-D ®
CONGESTION
Pseudoephedrine HCl 30 mg
Nasal Decongestant
METH BLOCKING TAREX® TECHNOLOGY ™
Non-Drowsy
Relieves
Nasal & Sinus Congestion
Sinus Pressure
Easy-to-take softgels.
Individually sealed.
Actual Size
Fast-acting nasal decongestant goes to work in as little as 30 minutes. With advanced meth-blocking technology for safer communities.
24 softgels
-
INGREDIENTS AND APPEARANCE
ZEPHREX D
pseudoephedrine hydrochloride capsule, gelatin coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0113-0401 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GUAR GUM (UNII: E89I1637KE) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color RED Score no score Shape OVAL Size 17mm Flavor Imprint Code ZD3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0113-0401-62 24 in 1 CARTON 04/17/2017 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:0113-0401-67 48 in 1 CARTON 05/08/2017 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/04/2016 Labeler - L. Perrigo Company (006013346)