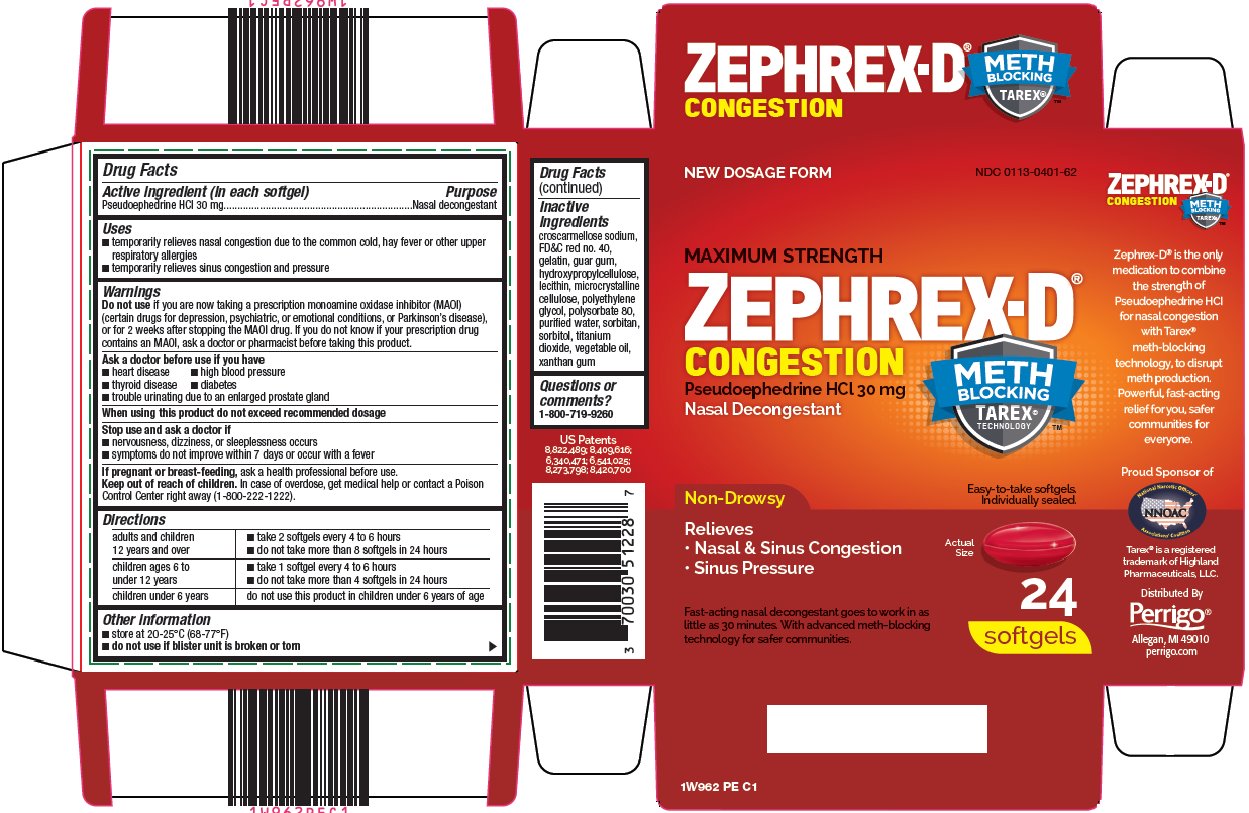
Uses
- •
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- •
- temporarily relieves sinus congestion and pressure
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
Stop use and ask a doctor if
- •
- nervousness, dizziness, or sleeplessness occurs
- •
- symptoms do not improve within 7 days or occur with a fever
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
|
adults and children 12 years and over |
|
|
children ages 6 to under 12 years |
|
|
children under 6 years |
do not use this product in children under 6 years of age |
Inactive ingredients
croscarmellose sodium, FD&C red no. 40, gelatin, guar gum, hydroxypropylcellulose, lecithin, microcrystalline cellulose, polyethylene glycol, polysorbate 80, purified water, sorbitan, sorbitol, titanium dioxide, vegetable oil, xanthan gum
Package/Label Principal Display Panel
NEW DOSAGE FORM
MAXIMUM STRENGTH
ZEPHREX-D ®
CONGESTION
Pseudoephedrine HCl 30 mg
Nasal Decongestant
METH BLOCKING TAREX® TECHNOLOGY ™
Non-Drowsy
Relieves
Nasal & Sinus Congestion
Sinus Pressure
Easy-to-take softgels.
Individually sealed.
Actual Size
Fast-acting nasal decongestant goes to work in as little as 30 minutes. With advanced meth-blocking technology for safer communities.
24 softgels