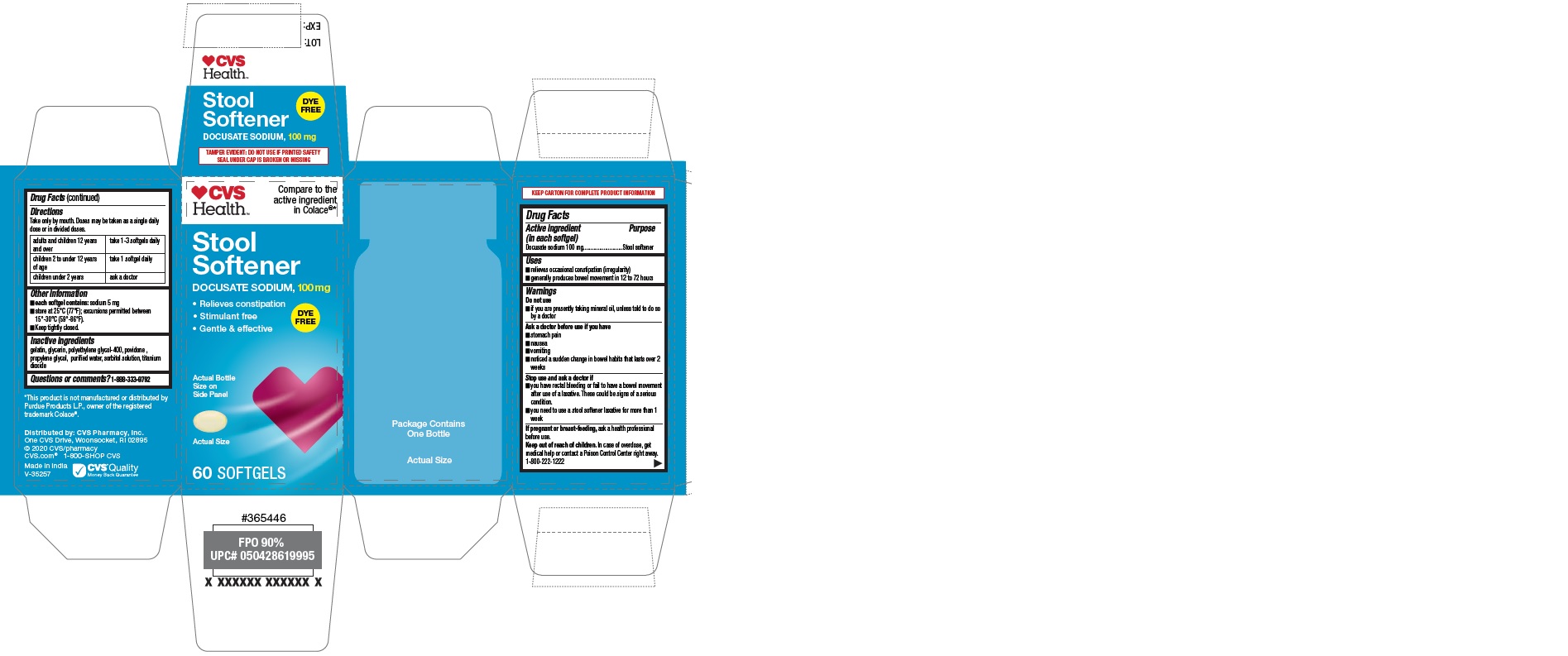
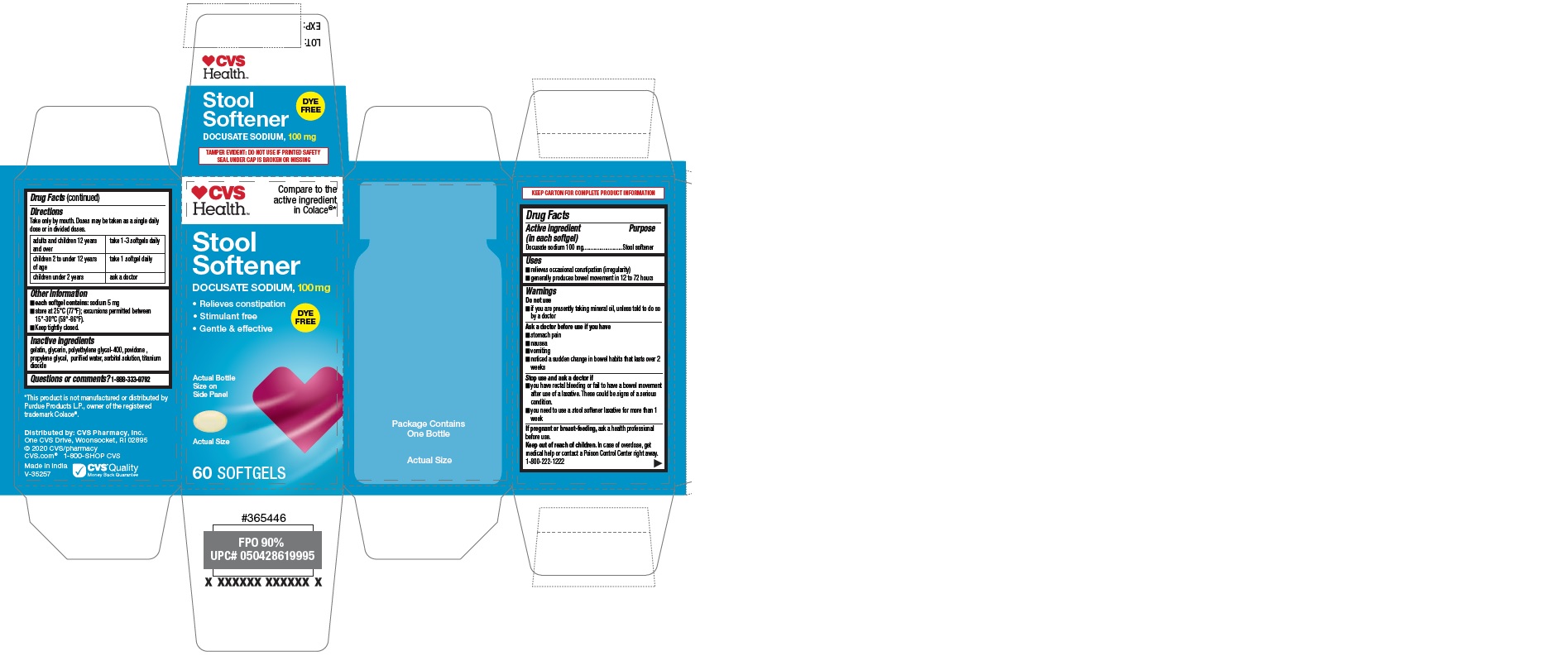
Label: CVS HEALTH STOOL SOFTENER CLEAR- docusate sodium capsule
- NDC Code(s): 69842-633-60
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each softgel)
- Purpose
- Uses
- Warnings
- ASK DOCTOR/PHARMACIST
- DO NOT USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS HEALTH STOOL SOFTENER CLEAR
docusate sodium capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-633 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POVIDONE K30 (UNII: U725QWY32X) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) GELATIN (UNII: 2G86QN327L) Product Characteristics Color white (natural color) Score no score Shape OVAL Size 12mm Flavor Imprint Code 100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-633-60 1 in 1 CARTON 02/11/2020 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 02/11/2020 Labeler - CVS Pharmacy (062312574)