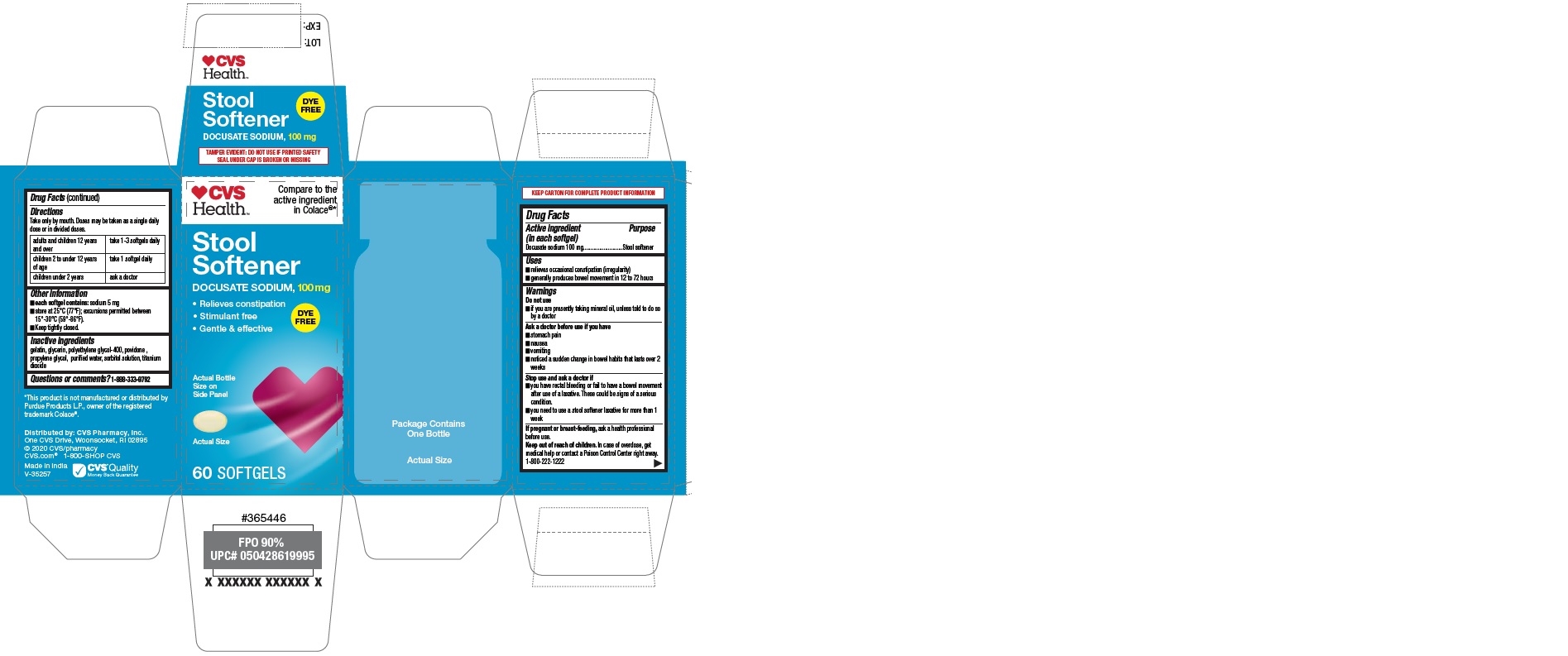
Uses
■ relieves occasional constipation (irregularity)
■ generally produces bowel movement in 12 to 72 hours
Ask a doctor before use if you have
■ stomach pain ■ nausea ■ vomiting
■ noticed a sudden change in bowel habits that lasts over
2 weeks
Stop use and ask a doctor if
■ you have rectal bleeding or fail to have a bowel
movement after use of a laxative. These could be signs
of a serious condition.
■ you need to use a stool softener laxative for more than
1 week
Keep out of reach of children. In case of overdose, get
medical help or contact a Poison Control Center right
away. 1-800-222-1222
Directions
Take only by mouth. Doses may be taken as a single daily
dose or in divided doses.
| adults and children 12 years
and over | take 1 to 3 softgels
daily |
| children 2 to under 12 years
of age | take 1 softgel
daily |
| children under 2 years | ask a doctor |
Other information
■
each softgel contains: sodium 5 mg
■ store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F).
■ Keep tightly closed
Inactive ingredients
gelatin, glycerin, polyethylene glycol-400, povidone (PVP K-30), propylene glycol, purified water, sorbitol solution, titanium dioxide
PRINCIPAL DISPLAY PANEL
CVS Health
Compare to the
active ingredient
in Colace®*
Stool
Softener
DOCUSATE SODIUM, 100 mg
•Relieves constipation
- Stimulant free
- Gentle & effective
DYE
FREE
60 SOFTGELS
*This product is not manufactured or
distributed by Purdue Products L.P., owner
of the registered trademark Colace®.