Label: ACUTE-KARE HEALTHCARE PERSONNEL HANDWASH- chloroxylenol soap
- NDC Code(s): 11084-800-41
- Packager: SC Johnson Professional USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product do not get it in the eyes; this product causes eye irritation upon direct contact. In case of eye exposure, rinse thoroughly with water. If eye irritation persists, contact a physician.
- Directions
- Inactive ingredients
- Questions or comments?
-
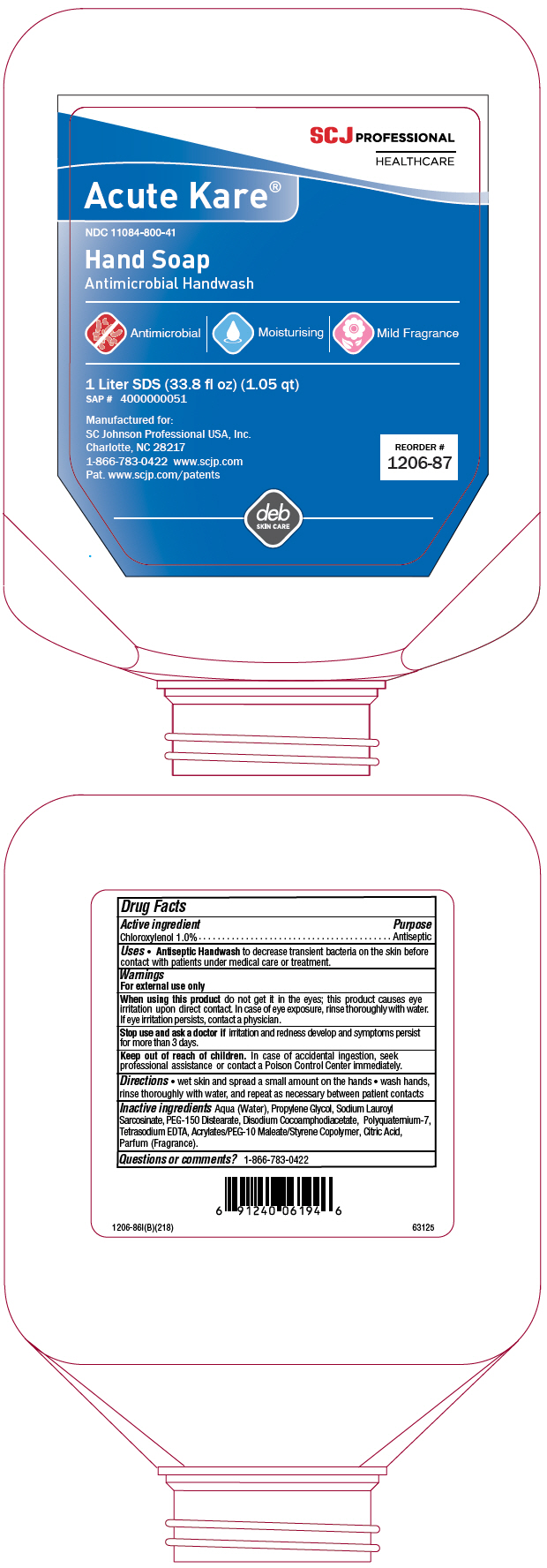
PRINCIPAL DISPLAY PANEL - 1 Liter Bottle Label
SCJ PROFESSIONAL
HEALTHCAREAcute Kare®
NDC 11084-800-41
Hand Soap
Antimicrobial HandwashAntimicrobial | Moisturising | Mild Fragrance
1 Liter SDS (33.8 fl oz) (1.05 qt)
SAP # 4000000051Manufactured for:
SC Johnson Professional USA, Inc.
Charlotte, NC 28217
1-866-783-0422 www.scjp.com
Pat. www.scjp.com/patentsREORDER #
1206-87deb
SKIN CARE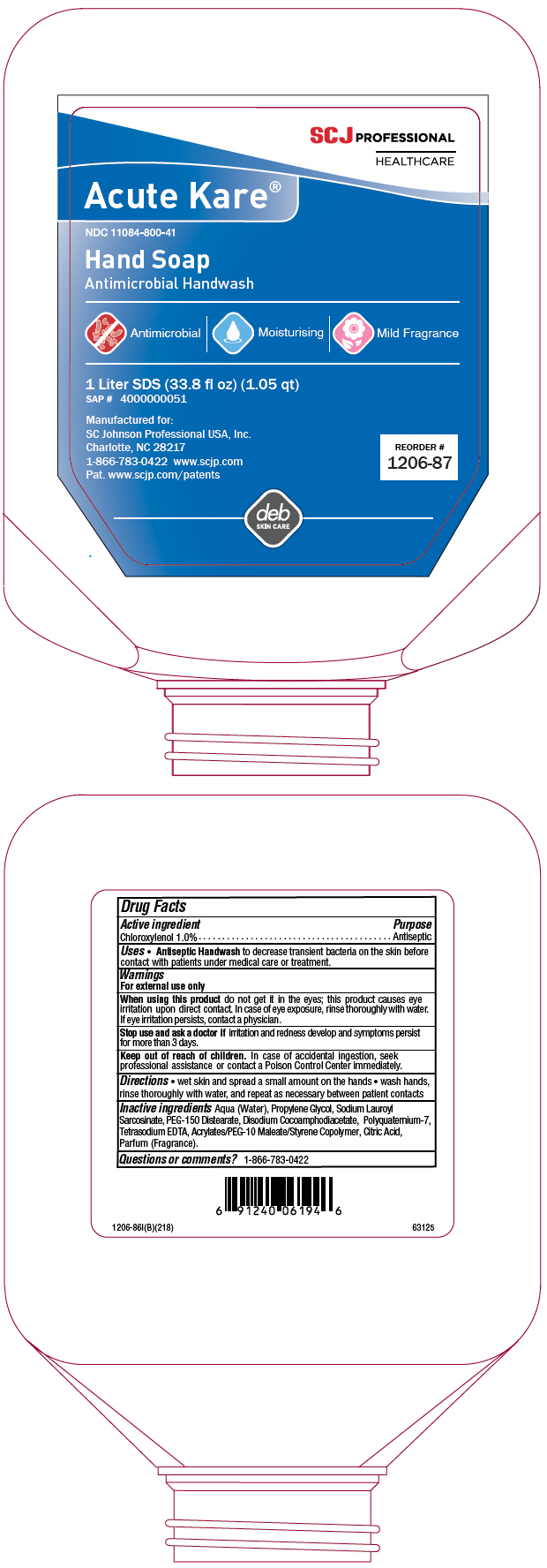
-
INGREDIENTS AND APPEARANCE
ACUTE-KARE HEALTHCARE PERSONNEL HANDWASH
chloroxylenol soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11084-800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600000 MW) (UNII: 0L414VCS5Y) EDETATE SODIUM (UNII: MP1J8420LU) METHACRYLATE/METHOXY PEG-10 MALEATE/STYRENE COPOLYMER (UNII: 39DK5WQ2PR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11084-800-41 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug 505G(a)(3) 02/01/2018 Labeler - SC Johnson Professional USA, Inc. (607378015) Establishment Name Address ID/FEI Business Operations Apex International Mfg LLC 015226132 MANUFACTURE(11084-800)