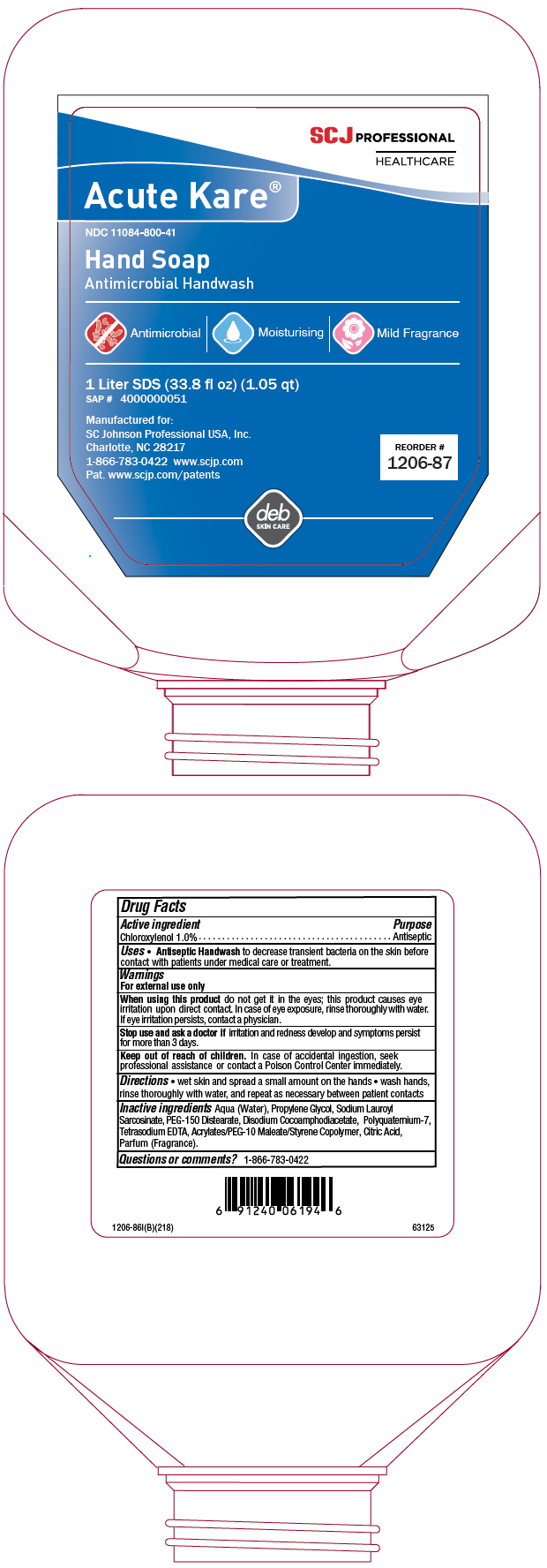
Uses
- Antiseptic Handwash to decrease transient bacteria on the skin before contact with patients under medical care or treatment.
Warnings
For external use only
When using this product do not get it in the eyes; this product causes eye irritation upon direct contact. In case of eye exposure, rinse thoroughly with water. If eye irritation persists, contact a physician.
Directions
- wet skin and spread a small amount on the hands
- wash hands, rinse thoroughly with water, and repeat as necessary between patient contacts
Inactive ingredients
Aqua (Water), Propylene Glycol, Sodium Lauroyl Sarcosinate, PEG-150 Distearate, Disodium Cocoamphodiacetate, Polyquaternium-7, Tetrasodium EDTA, Acrylates/PEG-10 Maleate/Styrene Copolymer, Citric Acid, Parfum (Fragrance).
PRINCIPAL DISPLAY PANEL - 1 Liter Bottle Label
SCJ PROFESSIONAL
HEALTHCARE
Acute Kare®
NDC 11084-800-41
Hand Soap
Antimicrobial Handwash
Antimicrobial | Moisturising | Mild Fragrance
1 Liter SDS (33.8 fl oz) (1.05 qt)
SAP # 4000000051
Manufactured for:
SC Johnson Professional USA, Inc.
Charlotte, NC 28217
1-866-783-0422 www.scjp.com
Pat. www.scjp.com/patents
REORDER #
1206-87
deb
SKIN CARE