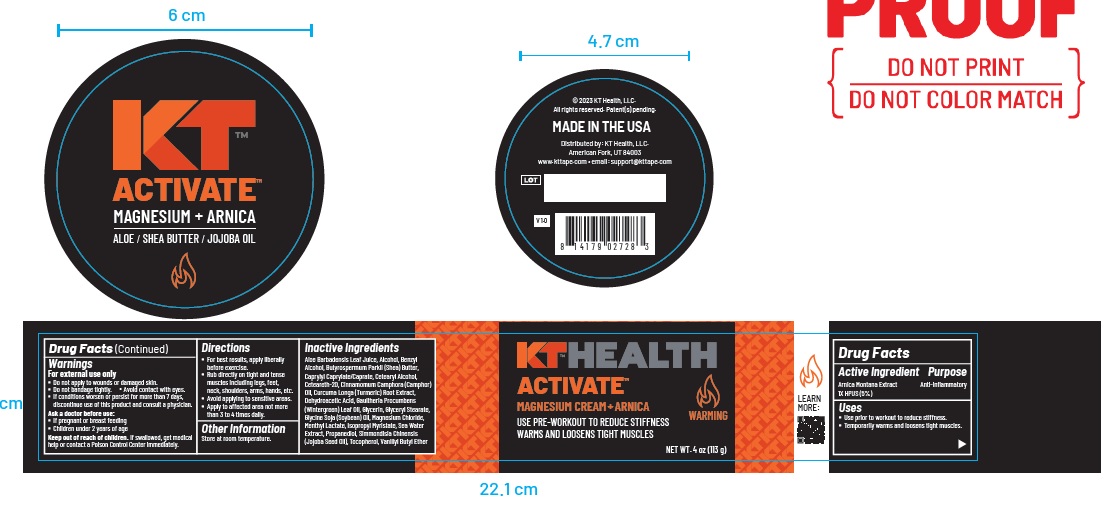
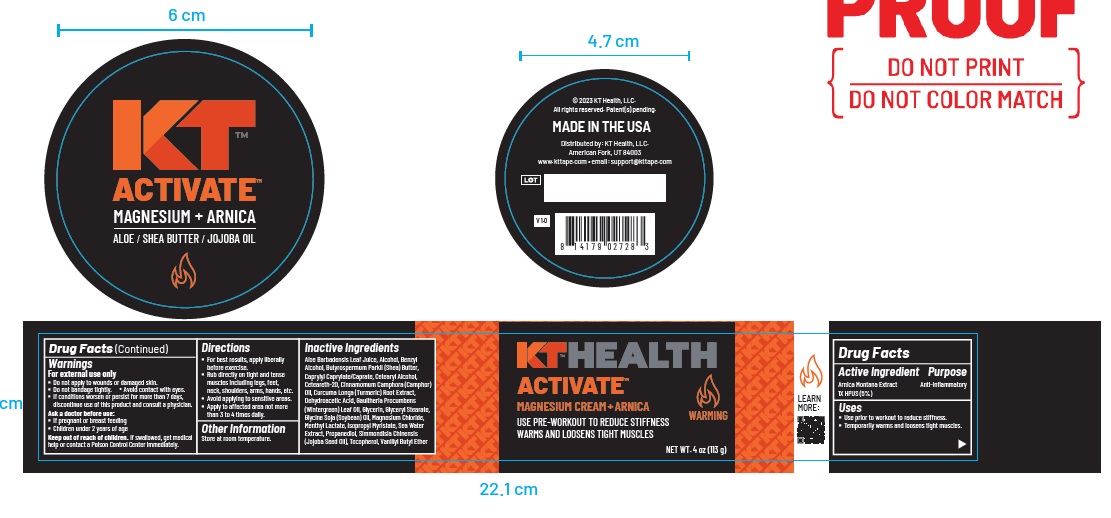
Label: KT ACTIVATE MAGNESIUM ARNICA- arnica montana whole cream
- NDC Code(s): 73044-105-01
- Packager: KT Health LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
Warnings
For external use only
- Do not apply to wounds or damaged skin.
- Do not bandage tightly. Avoid contact with eyes.
- If conditions worsen or persist for more than 7 days, discontinue use of this product and consult a physician.
Ask a doctor before use:
- If pregnant or breast feeding
- Children under 2 years of age
Keep out of reach of children. If swallowed, get medical
help or contact a Poison Control Center immediately. - KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Alcohol, Benzyl Alcohol, Butyrospermum Parkii (Shea) Butter, Caprylyl Caprylate/Caprate, Cetearyl Alcohol,
Ceteareth-20, Cinnamomum Camphora (Camphor) Oil, Curcuma Longa (Turmeric) Root Extract,Dehydroacetic Acid, Gaultheria Procumbens
(Wintergreen) Leaf Oil, Glycerin, Glyceryl Stearate, Glycine Soja (Soybean) Oil, Magnesium Chloride,Menthyl Lactate, Isopropyl Myristate, Sea Water Extract, Propanediol, Simmondisia Chinensis (Jojoba Seed Oil), Tocopherol, Vanillyl Butyl Ether - Other Information
- Product label
-
INGREDIENTS AND APPEARANCE
KT ACTIVATE MAGNESIUM ARNICA
arnica montana whole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73044-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA WHOLE (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA WHOLE 5 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) SHEA BUTTER (UNII: K49155WL9Y) CAPRYLYL CAPRYLATE/CAPRATE (UNII: 22MCG4RSMR) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CAMPHOR OIL (UNII: 75IZZ8Y727) TURMERIC (UNII: 856YO1Z64F) DEHYDROACETIC ACID (UNII: 2KAG279R6R) METHYL SALICYLATE (UNII: LAV5U5022Y) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL 1-STEARATE (UNII: 258491E1RZ) SOYBEAN OIL (UNII: 241ATL177A) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) METHYL LACTATE, (-)- (UNII: 0379G9C44S) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) SODIUM CHLORIDE (UNII: 451W47IQ8X) PROPANEDIOL (UNII: 5965N8W85T) SIMMONDSIN (UNII: O51H15R39K) JOJOBA OIL (UNII: 724GKU717M) TOCOPHEROL (UNII: R0ZB2556P8) VANILLYL BUTYL ETHER (UNII: S2ULN37C9R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73044-105-01 4 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/12/2023 Labeler - KT Health LLC (807008037)