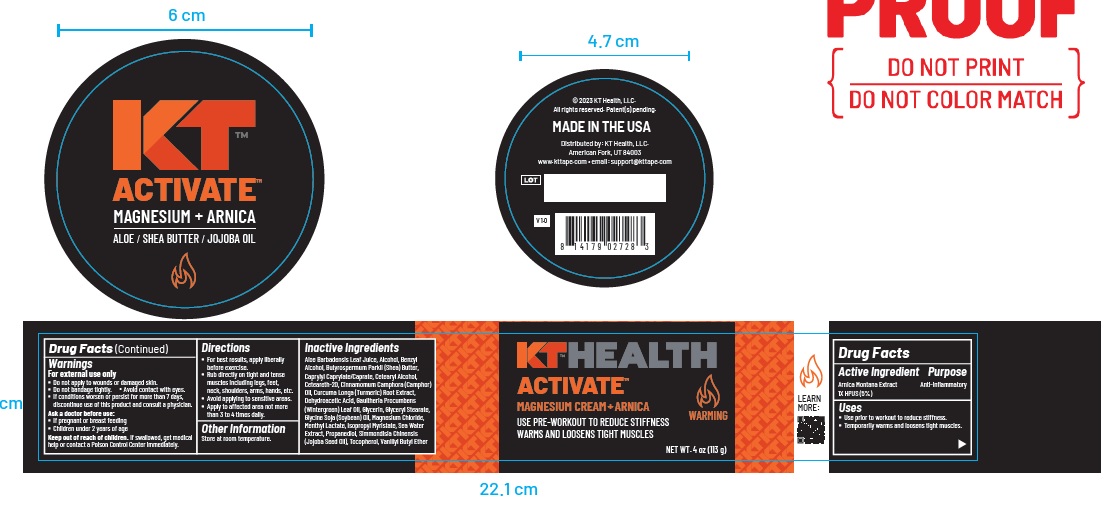
Warnings
For external use only
- Do not apply to wounds or damaged skin.
- Do not bandage tightly. Avoid contact with eyes.
- If conditions worsen or persist for more than 7 days, discontinue use of this product and consult a physician.
Ask a doctor before use:
- If pregnant or breast feeding
- Children under 2 years of age
Keep out of reach of children. If swallowed, get medical
help or contact a Poison Control Center immediately.
Directions
- For best results, apply liberally before exercise.
- Rub directly on tight and tense muscles including legs, feet, neck, shoulders, arms, hands, etc.
- Avoid applying to sensitive areas.
- Apply to affected area not more than 3 to 4 times daily.
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Alcohol, Benzyl Alcohol, Butyrospermum Parkii (Shea) Butter, Caprylyl Caprylate/Caprate, Cetearyl Alcohol,
Ceteareth-20, Cinnamomum Camphora (Camphor) Oil, Curcuma Longa (Turmeric) Root Extract,Dehydroacetic Acid, Gaultheria Procumbens
(Wintergreen) Leaf Oil, Glycerin, Glyceryl Stearate, Glycine Soja (Soybean) Oil, Magnesium Chloride,Menthyl Lactate, Isopropyl Myristate, Sea Water Extract, Propanediol, Simmondisia Chinensis (Jojoba Seed Oil), Tocopherol, Vanillyl Butyl Ether