Label: NASAL DECONGESTANT NON-DROWSY- pseudoephedrine hcl tablet, film coated
- NDC Code(s): 62011-0312-1, 62011-0312-2, 62011-0312-3
- Packager: Strategic Sourcing Services, LLC (Health Mart)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- diabetes
- heart disease
- high blood pressure
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
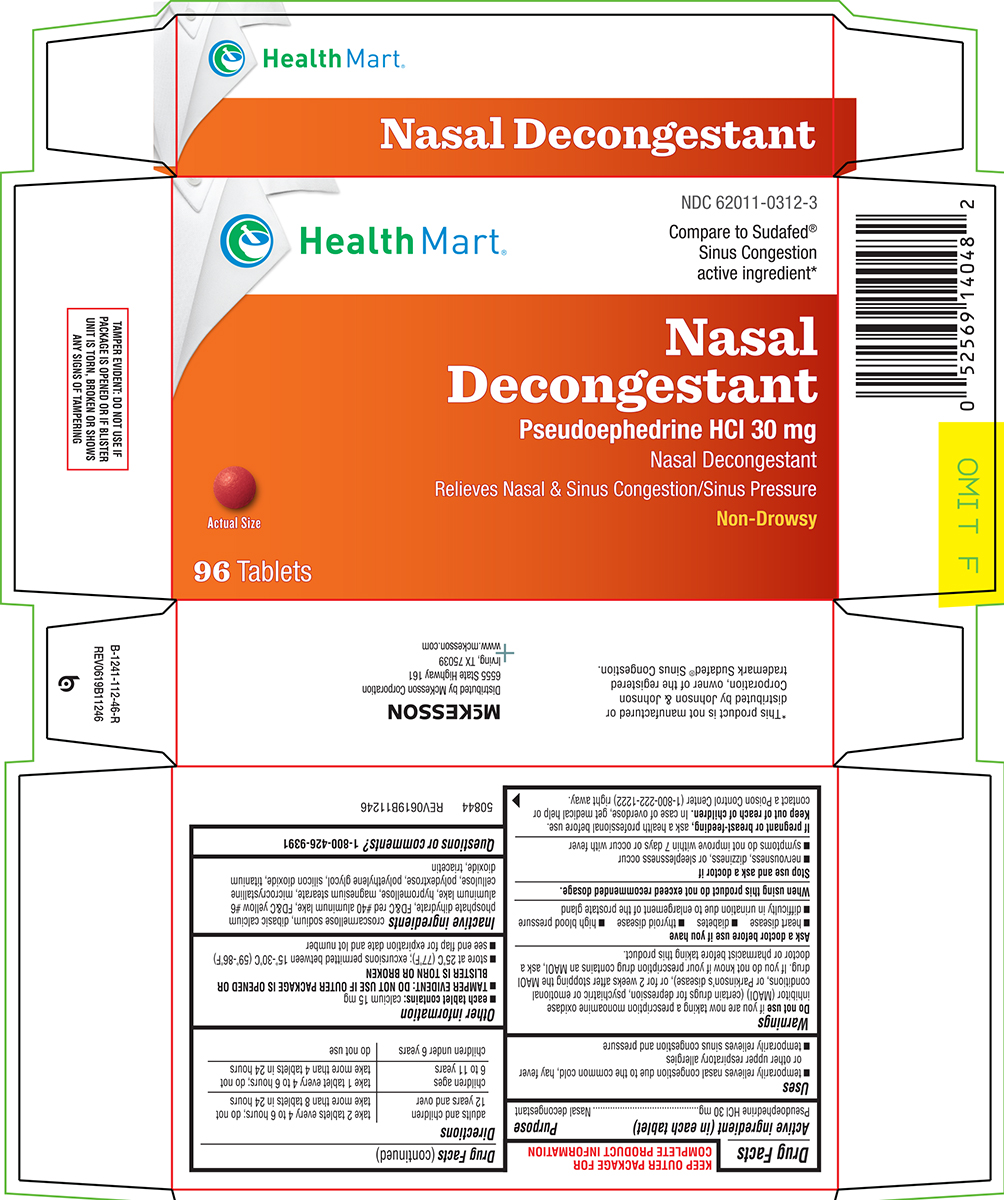
Principal display panel
Health Mart®
NDC 62011-0312-3
Compare to Sudafed®
Sinus Congestion
active ingredient*Nasal
Decongestant
Pseudoephedrine HCl 30 mg
Nasal Decongestant
Relieves Nasal & Sinus Congestion/Sinus PressureNon-Drowsy
Actual Size
96 Tablets
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING*This product is not manufactured or
distributed by Johnson & Johnson
Corporation, owner of the registered
trademark Sudafed® Congestion.
50844 REV0619B11246
Mckesson
Distributed by McKesson Corporation
6555 State Highway 161
Irving, TX 75039
www.mckesson.com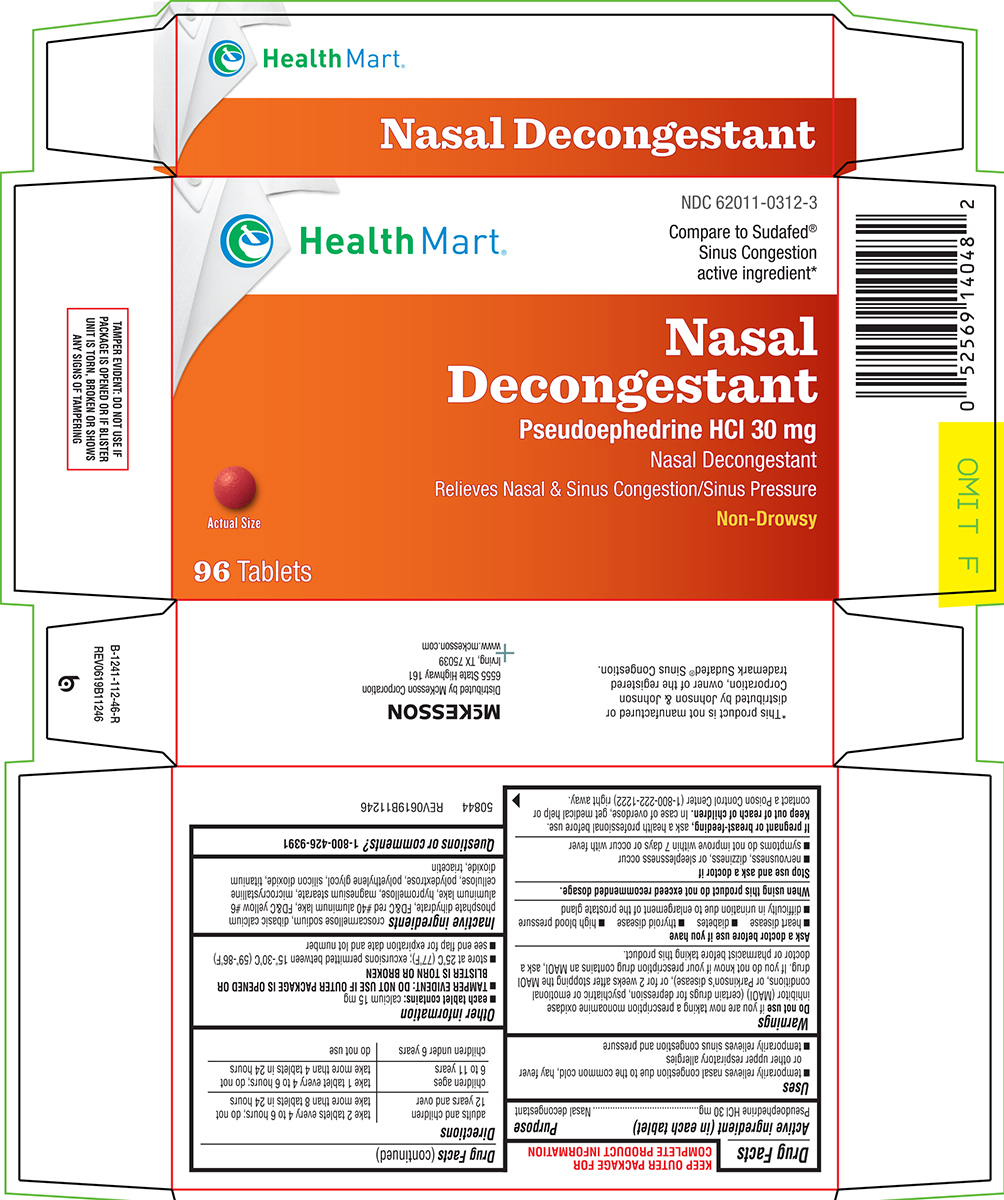
Healthmart 44-112
-
INGREDIENTS AND APPEARANCE
NASAL DECONGESTANT NON-DROWSY
pseudoephedrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62011-0312 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color red Score no score Shape ROUND Size 7mm Flavor Imprint Code 44;112 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62011-0312-1 1 in 1 CARTON 08/25/1981 08/12/2025 1 24 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:62011-0312-2 2 in 1 CARTON 08/25/1981 08/12/2025 2 24 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:62011-0312-3 4 in 1 CARTON 08/25/1981 08/12/2025 3 24 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/25/1981 08/12/2025 Labeler - Strategic Sourcing Services, LLC (Health Mart) (116956644) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(62011-0312) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 pack(62011-0312) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(62011-0312) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 pack(62011-0312) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(62011-0312)