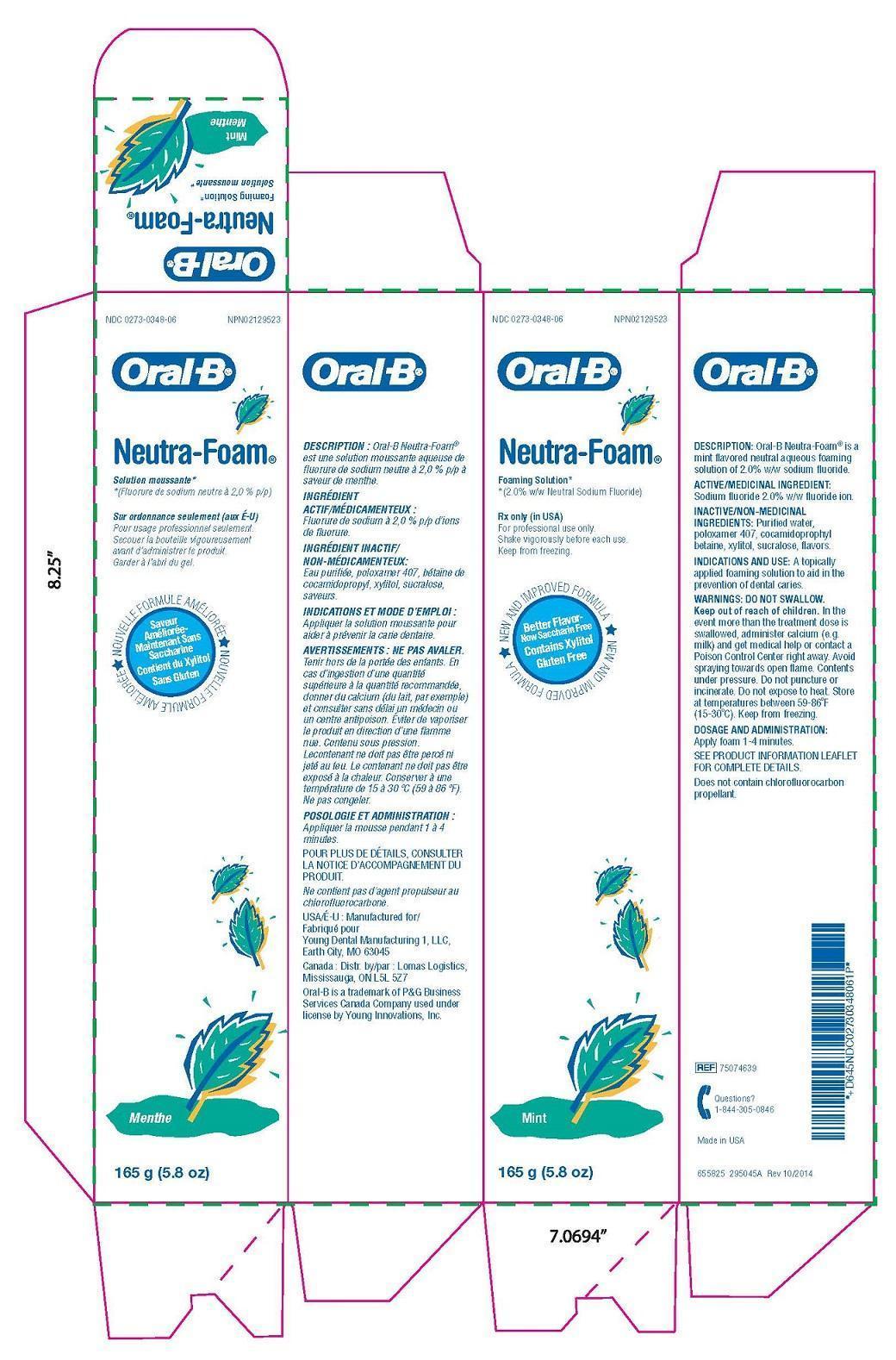
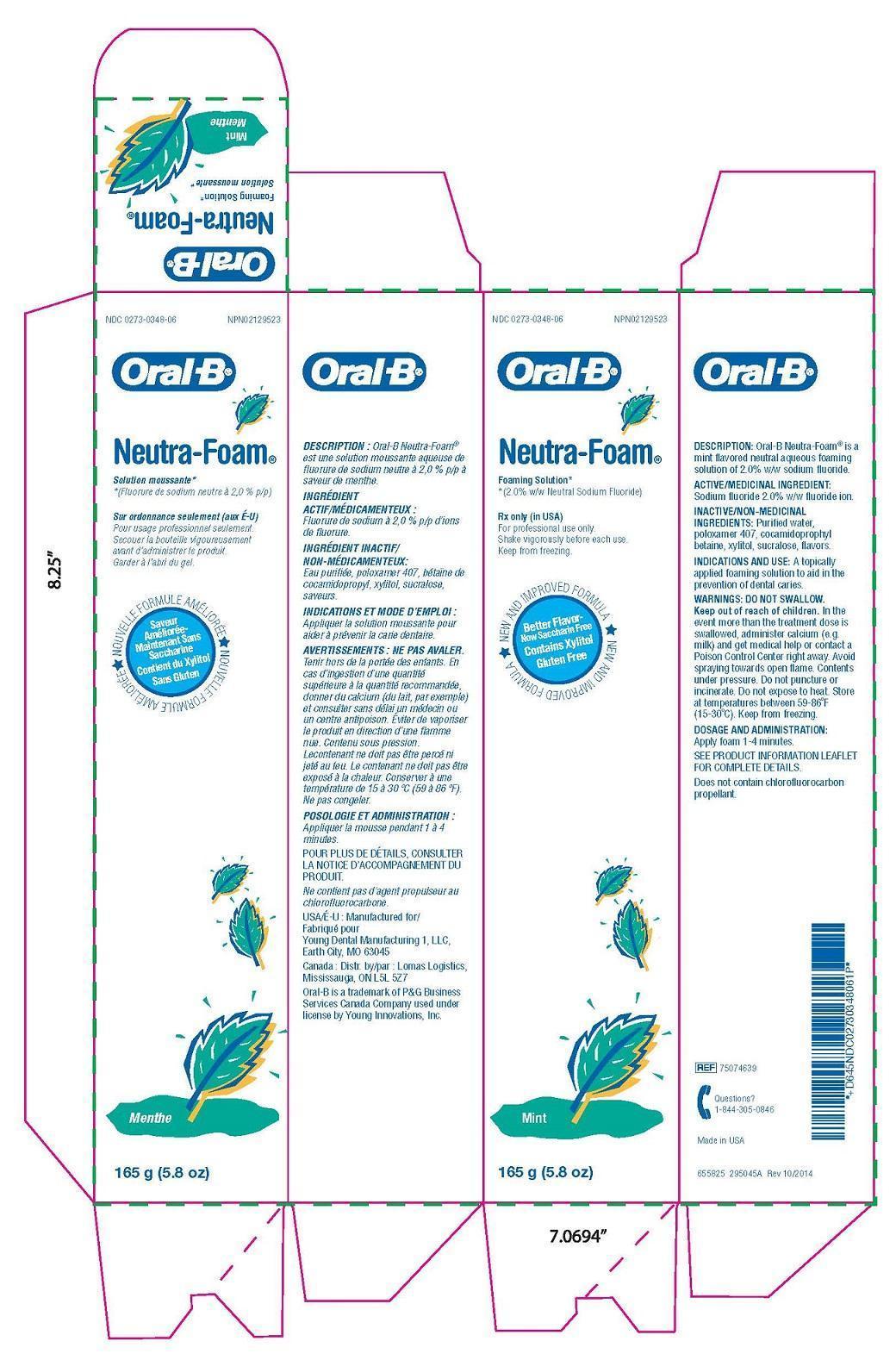
Label: ORAL B NEUTRA FOAM MINT MENTHE- sodium fluoride solution
- NDC Code(s): 0273-0348-06
- Packager: YOUNG DENTAL MANUFACTURING CO 1, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Descrption
- Inactives
- Indication and Usage
-
Warnings
In theevent more than the treatment dose isswallowed, administer calcium (e.g.milk) and get medical help or contact aPoison Control Center right away. Avoidspraying towards open flame. Contentsunder pressure. Do not puncture orincinerate. Do not expose to heat. Storeat temperatures between 59-86°F(15-30°C). Keep from freezing.
- Package Label
-
INGREDIENTS AND APPEARANCE
ORAL B NEUTRA FOAM MINT MENTHE
sodium fluoride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0273-0348 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) POLOXAMER 407 (UNII: TUF2IVW3M2) XYLITOL (UNII: VCQ006KQ1E) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor SPEARMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0273-0348-06 165 g in 1 TUBE; Type 0: Not a Combination Product 10/08/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/08/2015 Labeler - YOUNG DENTAL MANUFACTURING CO 1, LLC (006309355)