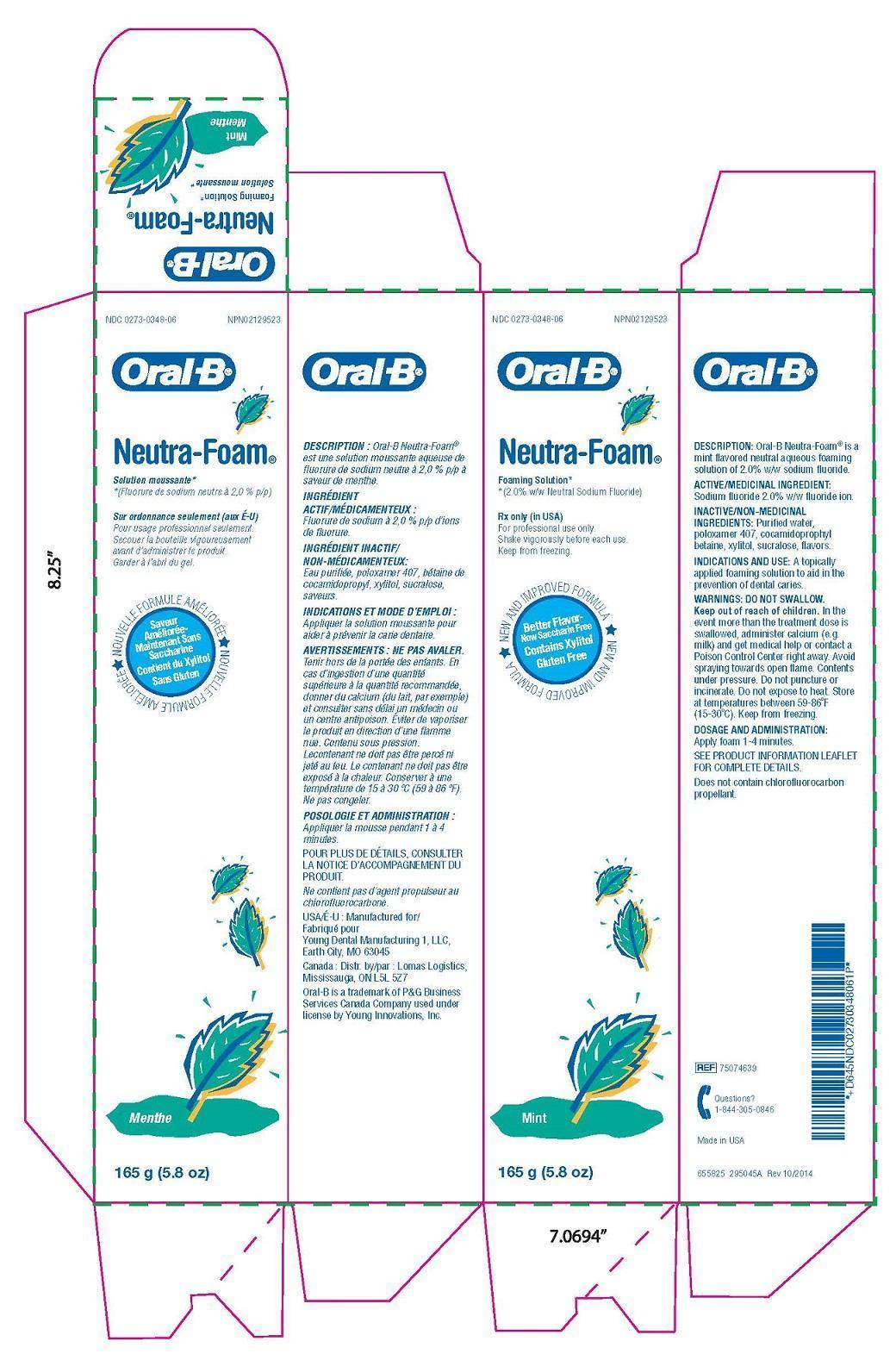
Descrption
Oral-B Neutra-Foam ® is a mint flavored neutral aqueous foaming solution of 2.0% w/w sodium fluoride.
Indication and Usage
A topically applied foaming solution to aid in the prevention of dental caries.
Warnings
In theevent more than the treatment dose isswallowed, administer calcium (e.g.milk) and get medical help or contact aPoison Control Center right away. Avoidspraying towards open flame. Contentsunder pressure. Do not puncture orincinerate. Do not expose to heat. Storeat temperatures between 59-86°F(15-30°C). Keep from freezing.