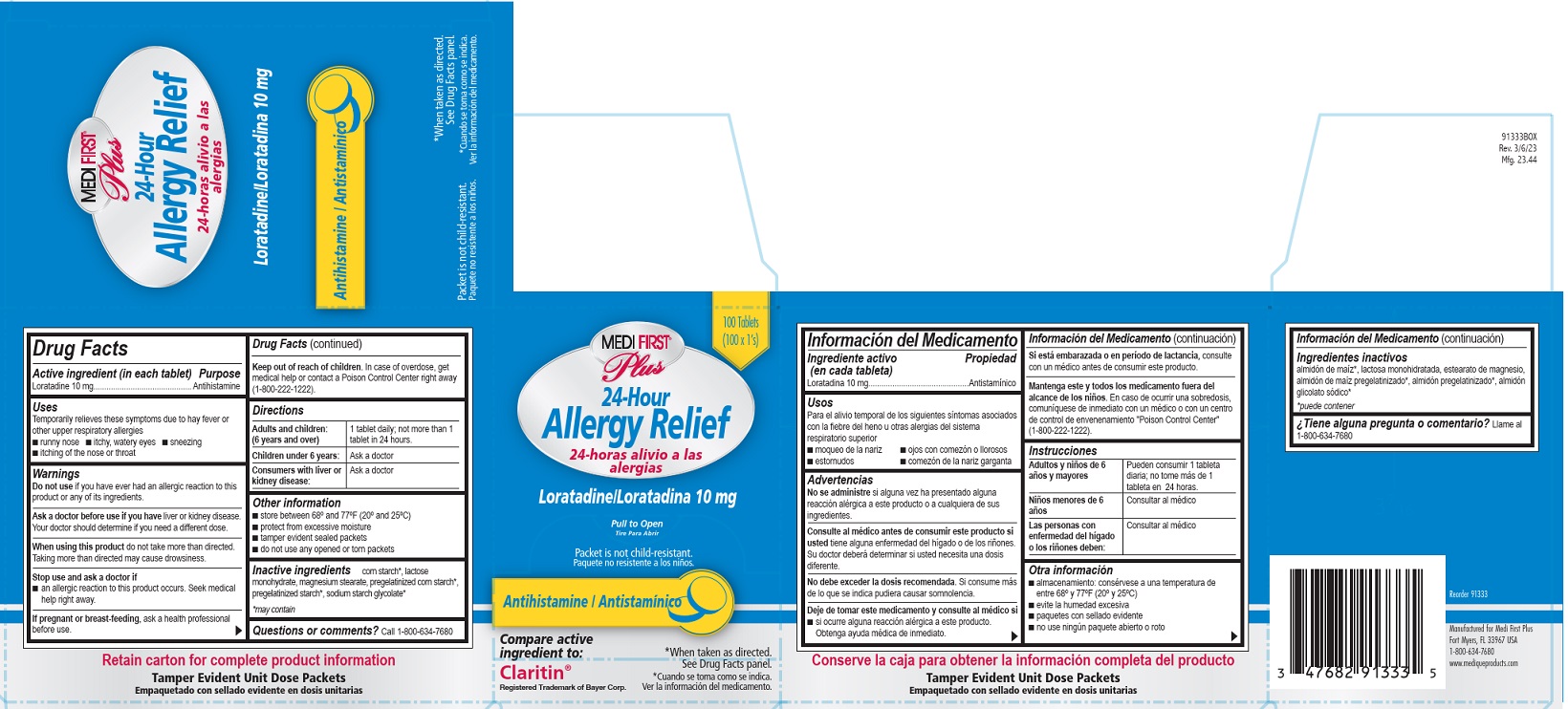
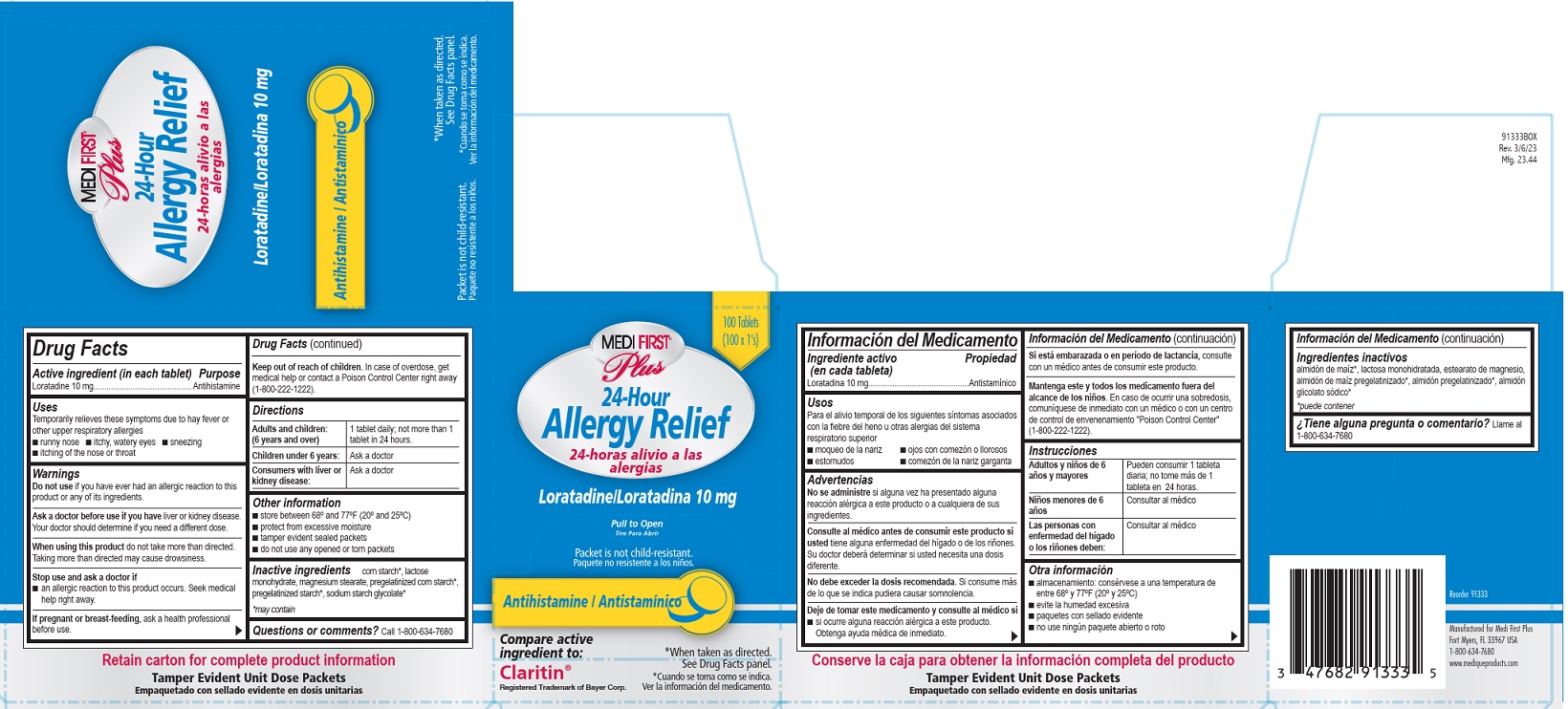
Label: MEDI FIRST PLUS ALLERGY RELIEF- loratadine tablet, film coated
MEDIQUE LORADAMED- loratadine tablet, film coated
-
NDC Code(s):
47682-203-12,
47682-203-46,
47682-203-50,
47682-913-33, view more47682-913-46
- Packager: Unifirst First Aid Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Ask a doctor before use if you have liver or kidney disease.
Your doctor should determine if you need a different dose.
When using this product do not take more than directed.
Taking more than directed may cause drowsiness.
- DOSAGE & ADMINISTRATION
- SPL UNCLASSIFIED SECTION
- INACTIVE INGREDIENT
- QUESTIONS
-
Medique Loradamed Label
Medique®
Loradamed
Antihistamine • Laratadine 10 mg
Antistaminico • Loratadina 10 mg
24-Hour Allergy Relief
24-horas alivio a las alergias
Pull to Open
Tire Para Abrir
packet not child-resistant.
Paquete no resistente a los ninos
*When taken as directed. See Drug Facts panel.
*Cuando se toma coma se indica. Ver la informacion del medicamento.
Collect Medibucks
See inside flap for more details
50 Tablets (50 x 1)

-
Medi-First Plus Allergy Relief Label
Medi-First® Plus
24-Hour Allergy Relief
24-horas alivo a las alergias
Laratadine/ Loratadina 10 mg
Pull to Open
Tire Para Abrir
Antihistamine/Antistaminico
Packet is not child-resistant.
Paquete no resistente a los ninos.
*When taken as directed. See Drug Facts panel.
*Cuando se toma coma se indica. Ver la informacion del medicamento.
Compare active ingredient to:
Claritin®
Registered Trademark of Bayer Corp.
-
INGREDIENTS AND APPEARANCE
MEDI FIRST PLUS ALLERGY RELIEF
loratadine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-913 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 6mm Flavor Imprint Code RX;526 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-913-33 100 in 1 BOX 04/23/2019 1 1 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:47682-913-46 1 in 1 PACKET; Type 0: Not a Combination Product 04/23/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076134 04/23/2019 MEDIQUE LORADAMED
loratadine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 6mm Flavor Imprint Code RX;526 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-203-50 50 in 1 BOX 12/30/2008 1 NDC:47682-203-46 1 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:47682-203-12 10 in 1 BOX 10/13/2016 2 NDC:47682-203-46 1 in 1 PACKET; Type 0: Not a Combination Product 3 NDC:47682-203-46 1 in 1 PACKET; Type 0: Not a Combination Product 12/30/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076134 12/30/2008 Labeler - Unifirst First Aid Corporation (832947092) Establishment Name Address ID/FEI Business Operations Prestige Packaging 080667761 pack(47682-203, 47682-913)