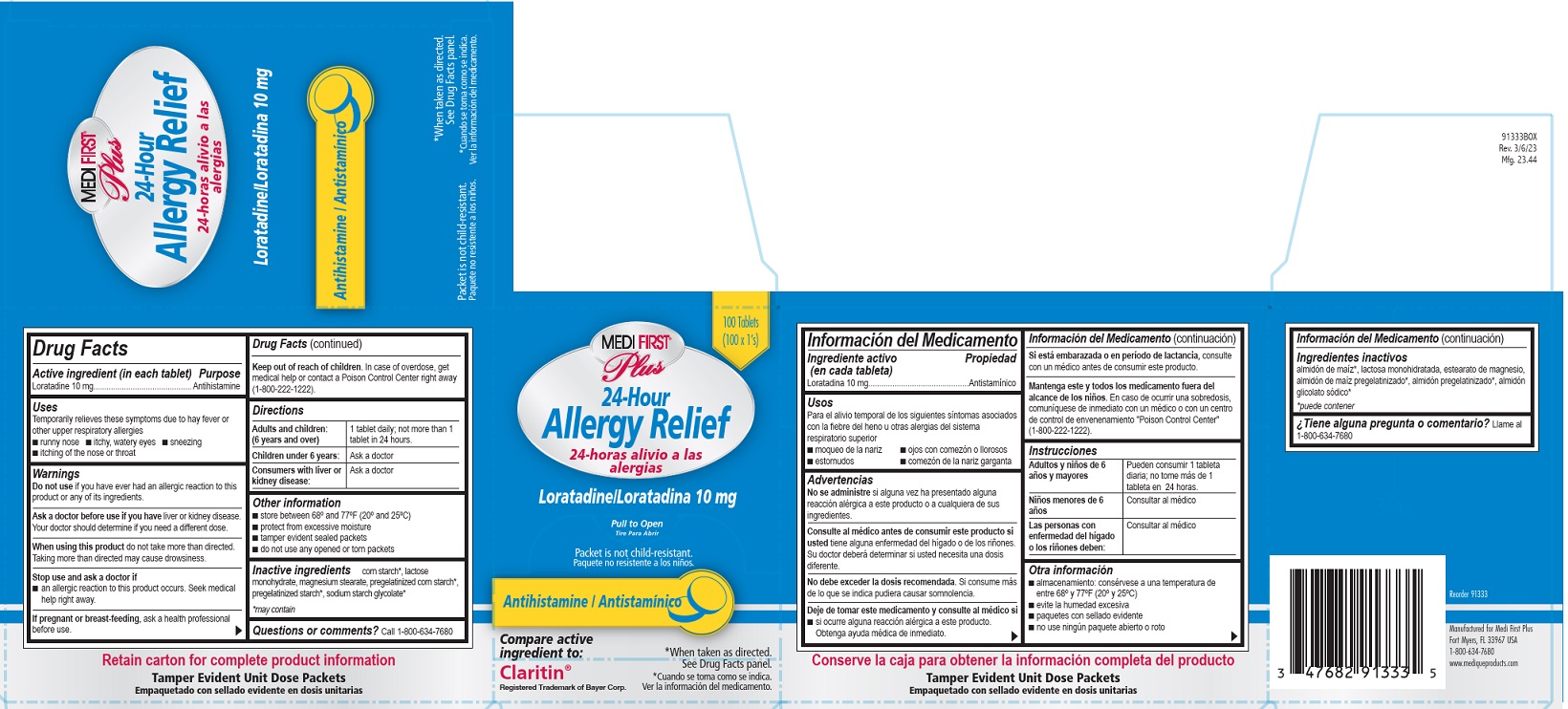
Uses
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have liver or kidney disease.
Your doctor should determine if you need a different dose.
When using this product do not take more than directed.
Taking more than directed may cause drowsiness.
Directions
Adults and children (6 years and over)
1 tablet daily, not more than 1 tablet in 24 hours.
Children under 6 years:
Ask a doctor
Consumer with liver or kidney disease:
Ask a doctor
Other information
- store between 68º and 77ºF (20º and 25ºC )
- protect from excessive moisture
- tamper evident sealed packets
- do not use any opened or torn packets
Inactive ingredients
corn starch*, lactose monohydrate, magnesium stearate, pregelatinized corn starch*, pregelatinized starch*, sodium starch glycolate*
* may contain
Medique Loradamed Label
Medique®
Loradamed
Antihistamine • Laratadine 10 mg
Antistaminico • Loratadina 10 mg
24-Hour Allergy Relief
24-horas alivio a las alergias
Pull to Open
Tire Para Abrir
packet not child-resistant.
Paquete no resistente a los ninos
*When taken as directed. See Drug Facts panel.
*Cuando se toma coma se indica. Ver la informacion del medicamento.
Collect Medibucks
See inside flap for more details
50 Tablets (50 x 1)

Medi-First Plus Allergy Relief Label
Medi-First® Plus
24-Hour Allergy Relief
24-horas alivo a las alergias
Laratadine/ Loratadina 10 mg
Pull to Open
Tire Para Abrir
Antihistamine/Antistaminico
Packet is not child-resistant.
Paquete no resistente a los ninos.
*When taken as directed. See Drug Facts panel.
*Cuando se toma coma se indica. Ver la informacion del medicamento.
Compare active ingredient to:
Claritin®
Registered Trademark of Bayer Corp.