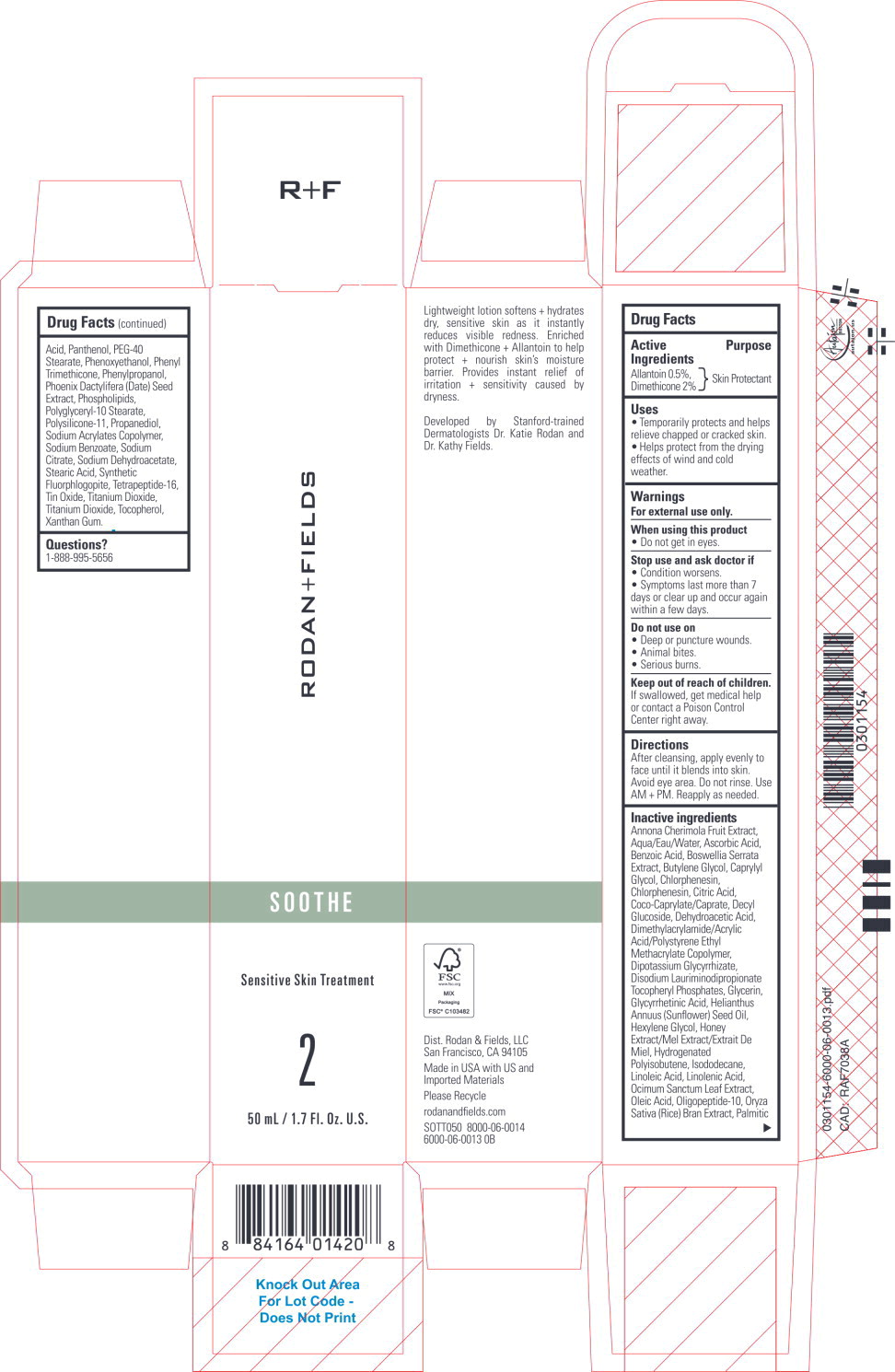
Label: SOOTHE SENSITIVE SKIN TREATMENT- allantoin, dimethicone lotion
- NDC Code(s): 14222-2420-1, 14222-2420-2, 14222-2420-3
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Annona Cherimola Fruit Extract, Aqua/Eau/Water, Ascorbic Acid, Benzoic Acid, Boswellia Serrata Extract, Butylene Glycol, Caprylyl Glycol, Chlorphenesin, Chlorphenesin, Citric Acid, Coco-Caprylate/Caprate, Decyl Glucoside, Dehydroacetic Acid, Dimethylacrylamide/Acrylic Acid/Polystyrene Ethyl Methacrylate Copolymer, Dipotassium Glycyrrhizate, Disodium Lauriminodipropionate Tocopheryl Phosphates, Glycerin, Glycyrrhetinic Acid, Helianthus Annuus (Sunflower) Seed Oil, Hexylene Glycol, Honey Extract/Mel Extract/Extrait De Miel, Hydrogenated Polyisobutene, Isododecane, Linoleic Acid, Linolenic Acid, Ocimum Sanctum Leaf Extract, Oleic Acid, Oligopeptide-10, Oryza Sativa (Rice) Bran Extract, Palmitic Acid, Panthenol, PEG-40 Stearate, Phenoxyethanol, Phenyl Trimethicone, Phenylpropanol, Phoenix Dactylifera (Date) Seed Extract, Phospholipids, Polyglyceryl-10 Stearate, Polysilicone-11, Propanediol, Sodium Acrylates Copolymer, Sodium Benzoate, Sodium Citrate, Sodium Dehydroacetate, Stearic Acid, Synthetic Fluorphlogopite, Tetrapeptide-16, Tin Oxide, Titanium Dioxide, Titanium Dioxide, Tocopherol, Xanthan Gum.
- Questions?
- Principal Display Panel – 50 mL Carton Label
- Principal Display Panel – 50 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
SOOTHE SENSITIVE SKIN TREATMENT
allantoin, dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Allantoin (UNII: 344S277G0Z) (Allantoin - UNII:344S277G0Z) Allantoin 0.5 g in 100 mL Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 2 g in 100 mL Inactive Ingredients Ingredient Name Strength CHERIMOYA (UNII: 33WVT714QS) ASCORBIC ACID (UNII: PQ6CK8PD0R) BENZOIC ACID (UNII: 8SKN0B0MIM) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) DEHYDROACETIC ACID (UNII: 2KAG279R6R) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) DISODIUM LAURIMINODIPROPIONATE TOCOPHERYL PHOSPHATES (UNII: 0K5Y9U1P6M) GLYCERIN (UNII: PDC6A3C0OX) ENOXOLONE (UNII: P540XA09DR) SUNFLOWER OIL (UNII: 3W1JG795YI) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HONEY (UNII: Y9H1V576FH) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) ISODODECANE (UNII: A8289P68Y2) LINOLEIC ACID (UNII: 9KJL21T0QJ) LINOLENIC ACID (UNII: 0RBV727H71) HOLY BASIL LEAF (UNII: SCJ765569P) OLEIC ACID (UNII: 2UMI9U37CP) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PALMITIC ACID (UNII: 2V16EO95H1) PANTHENOL (UNII: WV9CM0O67Z) PEG-40 STEARATE (UNII: ECU18C66Q7) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PHENYLPROPANOL (UNII: 0F897O3O4M) PHOENIX DACTYLIFERA SEED (UNII: 73NE6T0Q00) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-10 STEARATE (UNII: 90TF85HH91) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PROPANEDIOL (UNII: 5965N8W85T) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) STANNIC OXIDE (UNII: KM7N50LOS6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2420-1 1 in 1 CARTON 03/09/2021 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:14222-2420-2 1 in 1 CARTON 06/01/2021 2 10 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:14222-2420-3 2 mL in 1 PACKET; Type 0: Not a Combination Product 04/12/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/09/2021 Labeler - Rodan & Fields (051659584)