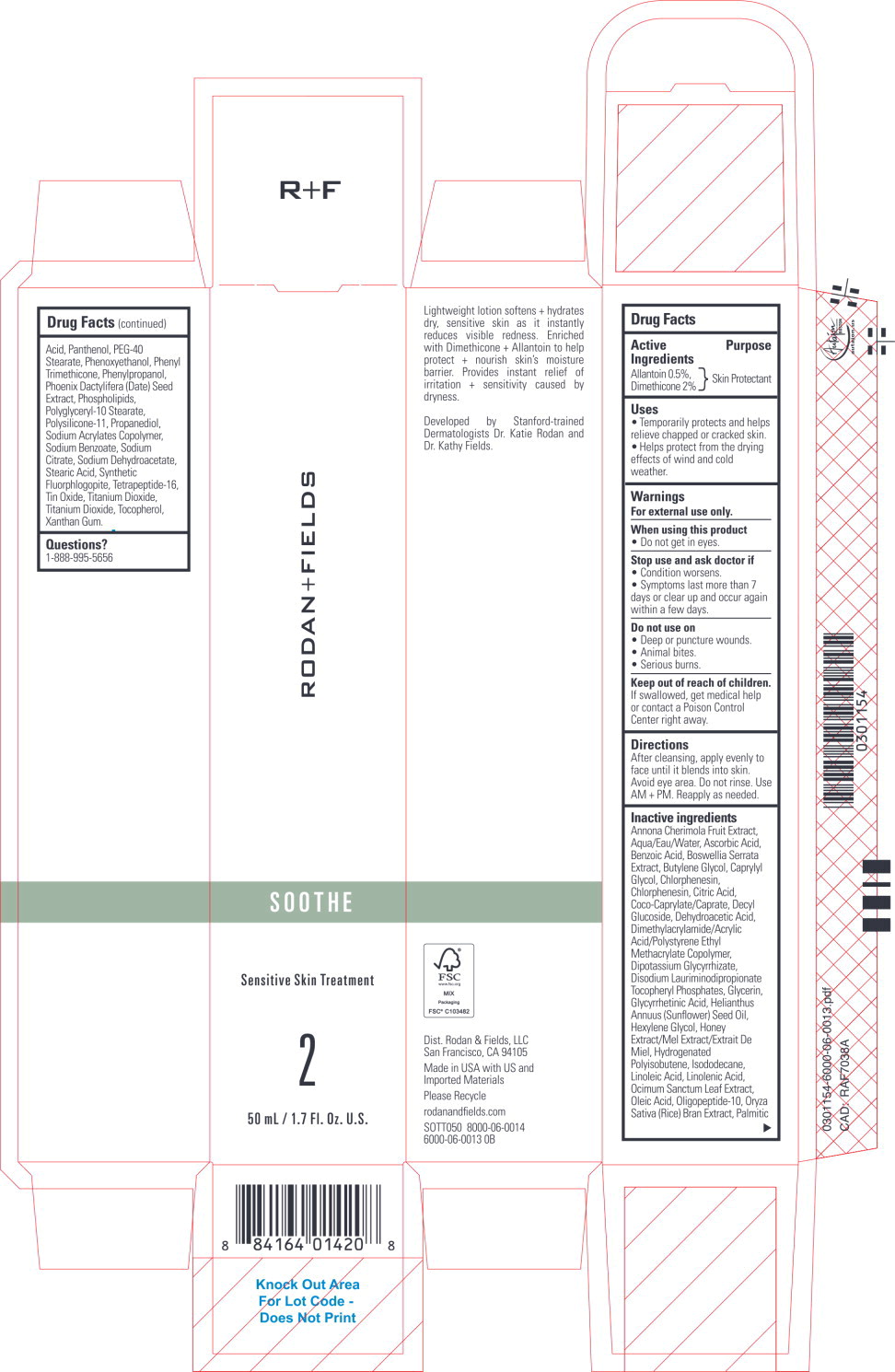
Uses
- Temporarily protects and helps relieve chapped or cracked skin.
- Helps protect from the drying effects of wind and cold weather.
Warnings
For external use only.
Directions
After cleansing, apply evenly to face until it blends into skin. Avoid eye area. Do not rinse. Use AM + PM. Reapply as needed.
Inactive ingredients
Annona Cherimola Fruit Extract, Aqua/Eau/Water, Ascorbic Acid, Benzoic Acid, Boswellia Serrata Extract, Butylene Glycol, Caprylyl Glycol, Chlorphenesin, Chlorphenesin, Citric Acid, Coco-Caprylate/Caprate, Decyl Glucoside, Dehydroacetic Acid, Dimethylacrylamide/Acrylic Acid/Polystyrene Ethyl Methacrylate Copolymer, Dipotassium Glycyrrhizate, Disodium Lauriminodipropionate Tocopheryl Phosphates, Glycerin, Glycyrrhetinic Acid, Helianthus Annuus (Sunflower) Seed Oil, Hexylene Glycol, Honey Extract/Mel Extract/Extrait De Miel, Hydrogenated Polyisobutene, Isododecane, Linoleic Acid, Linolenic Acid, Ocimum Sanctum Leaf Extract, Oleic Acid, Oligopeptide-10, Oryza Sativa (Rice) Bran Extract, Palmitic Acid, Panthenol, PEG-40 Stearate, Phenoxyethanol, Phenyl Trimethicone, Phenylpropanol, Phoenix Dactylifera (Date) Seed Extract, Phospholipids, Polyglyceryl-10 Stearate, Polysilicone-11, Propanediol, Sodium Acrylates Copolymer, Sodium Benzoate, Sodium Citrate, Sodium Dehydroacetate, Stearic Acid, Synthetic Fluorphlogopite, Tetrapeptide-16, Tin Oxide, Titanium Dioxide, Titanium Dioxide, Tocopherol, Xanthan Gum.