Label: DELSYM NIGHTTIME COUGH- acetaminophen, dextromethorphan hydrobromide, and triprolidine hydrochloride solution
- NDC Code(s): 63824-223-66
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
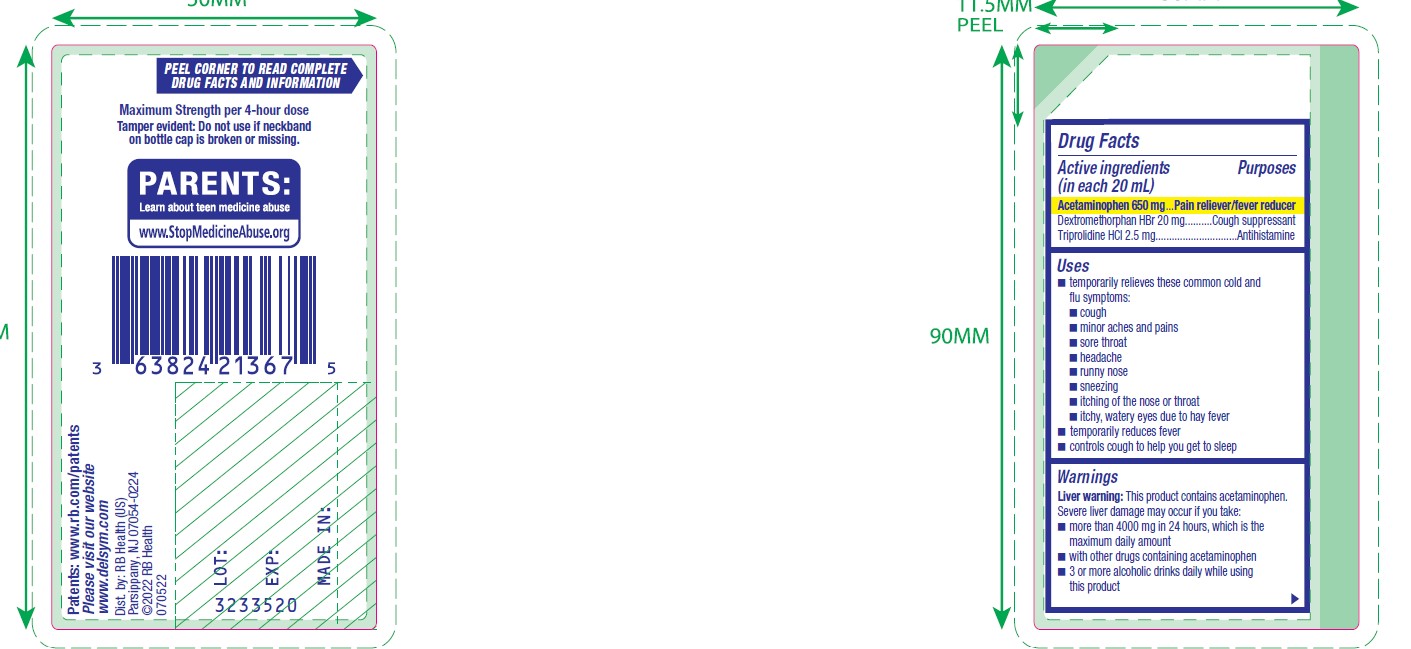
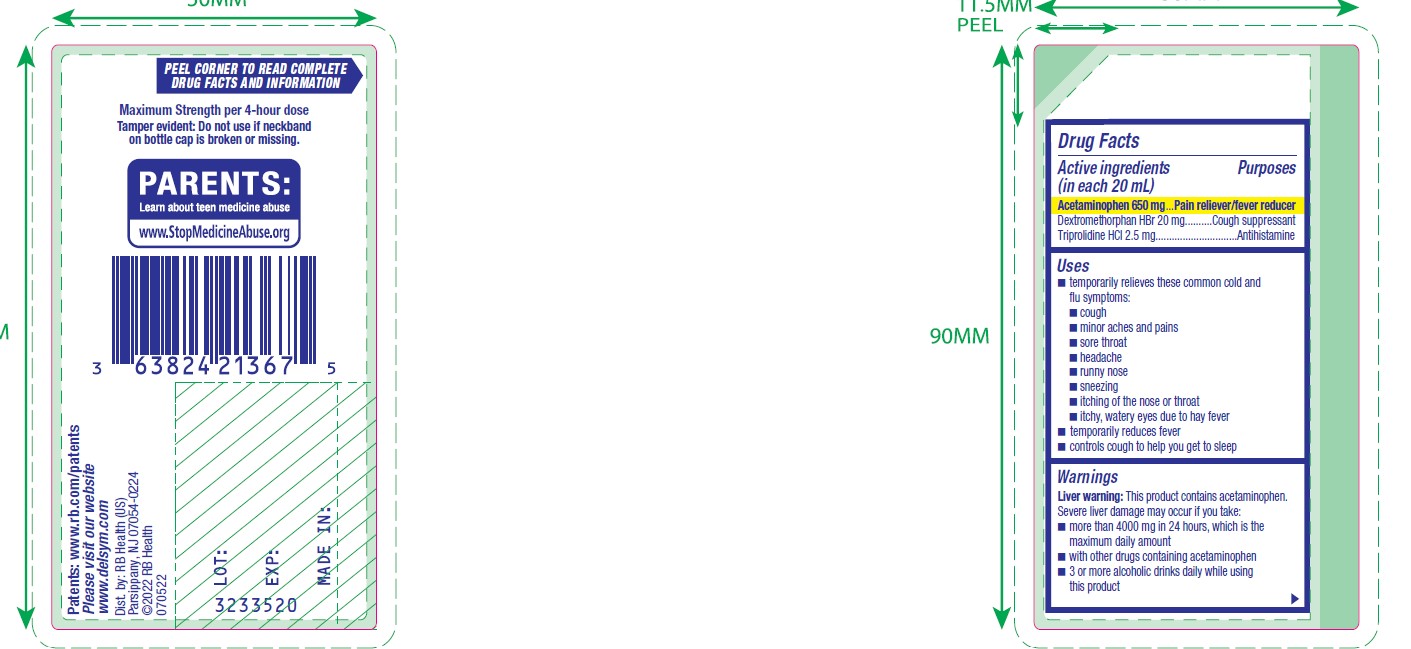
- ACTIVE INGREDIENT
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4000 mg in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
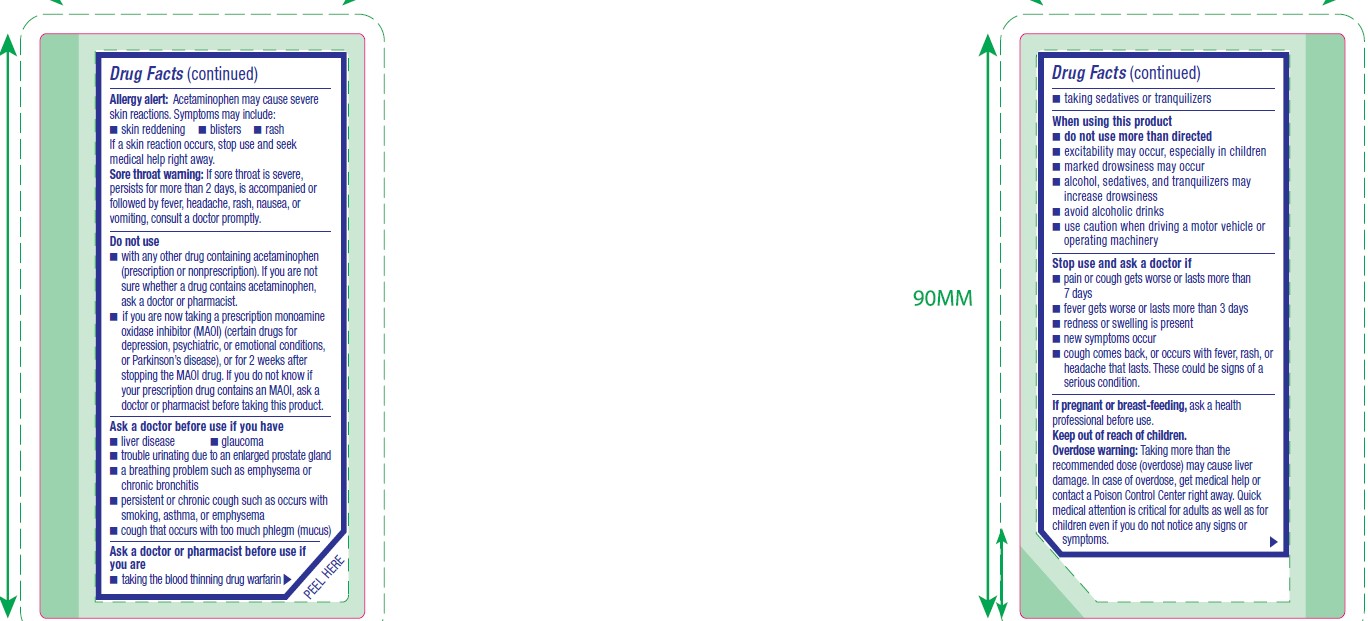
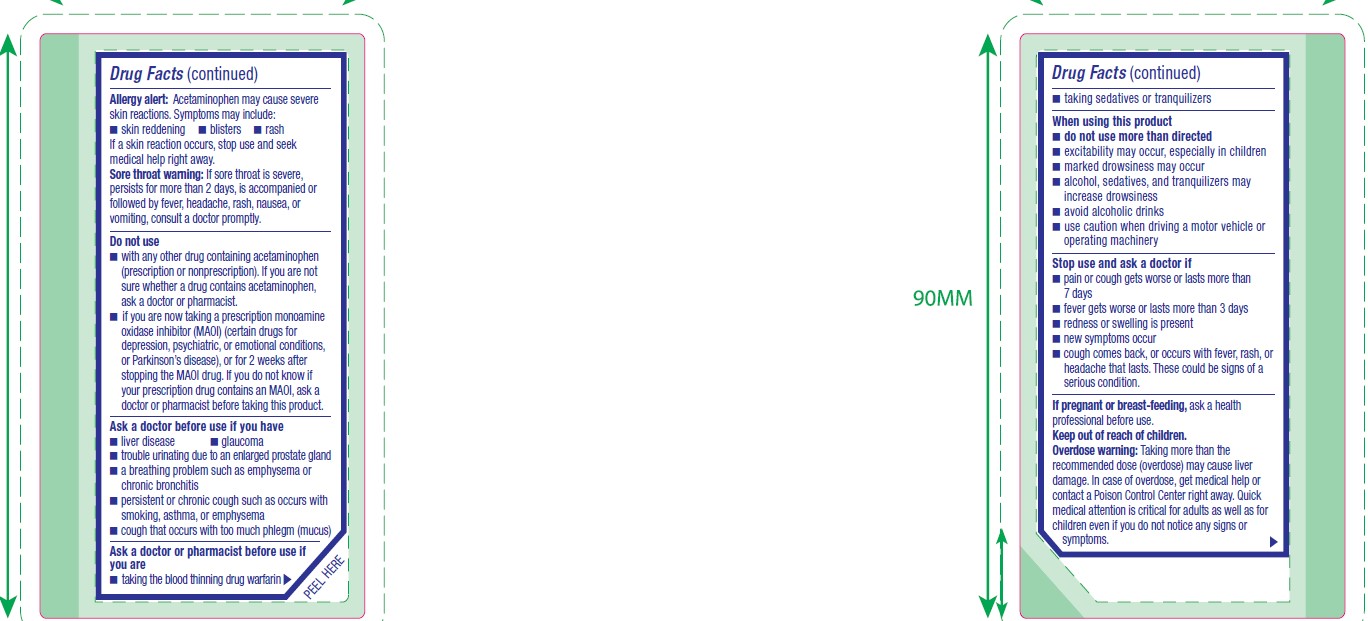
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not use more than directed
- excitability may occur, especially in children
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- use caution when driving a motor vehicle or operating machinery
- Overdose warning
-
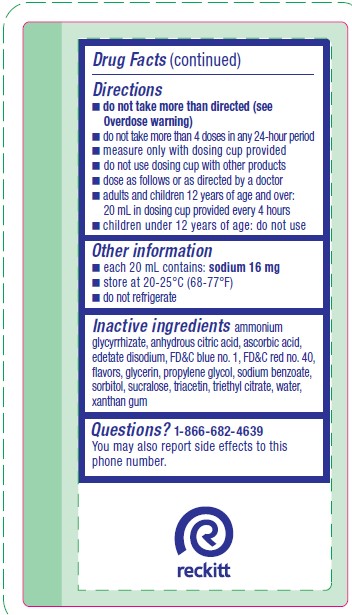
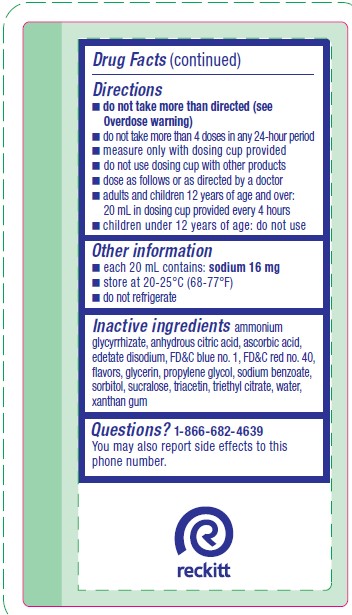
Directions
- do not take more than directed (see Overdose warning)
- do not take more than 4 doses in any 24-hour period
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by a doctor
- adults and children 12 years of age and over: 20 mL in dosing cup provided every 4 hours
- children under 12 years of age: do not use
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 180 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
DELSYM NIGHTTIME COUGH
acetaminophen, dextromethorphan hydrobromide, and triprolidine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-223 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 2.5 mg in 20 mL Inactive Ingredients Ingredient Name Strength AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ASCORBIC ACID (UNII: PQ6CK8PD0R) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color blue Score Shape Size Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-223-66 180 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 06/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/30/2020 Labeler - RB Health (US) LLC (081049410)