Label: COBROXIN FOR CHRONIC PAIN- naja naja venom gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 47219-104-50 - Packager: RECEPTOPHARM INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 3, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
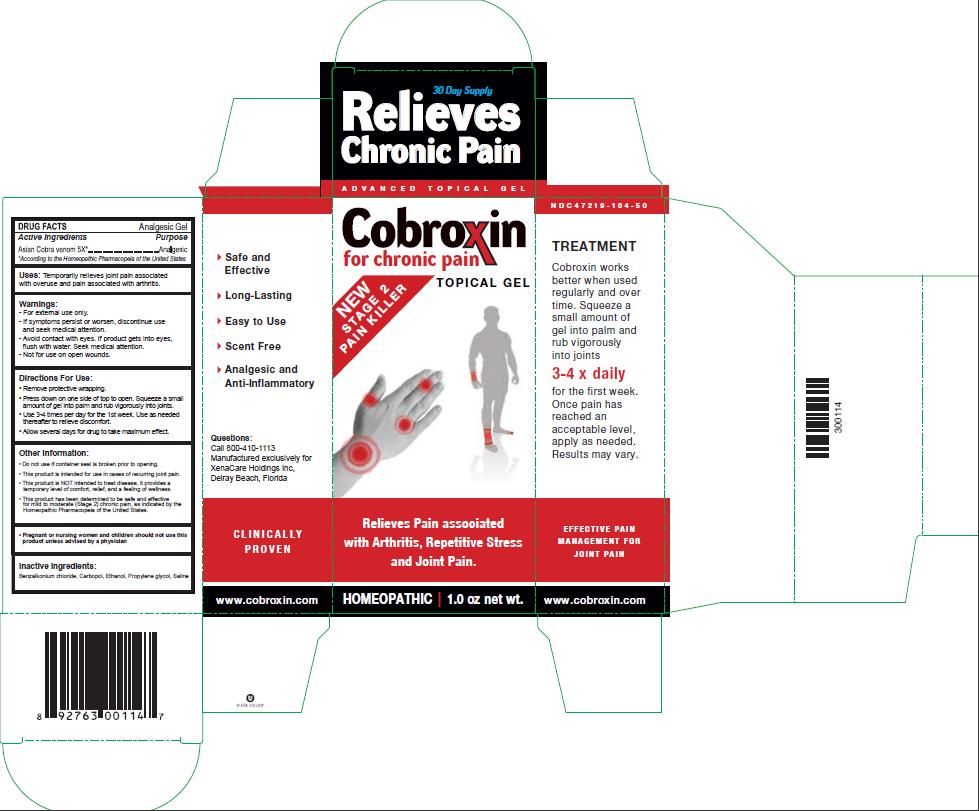
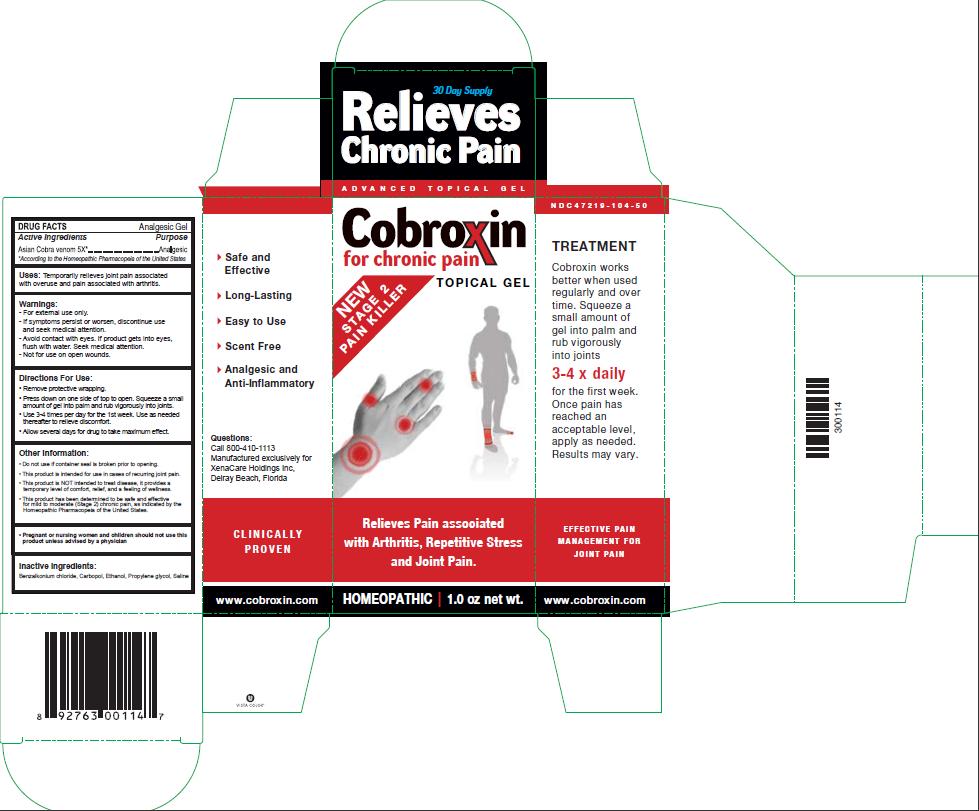
- Active Ingredients
- Purpose
- INDICATIONS & USAGE
- Warnings:
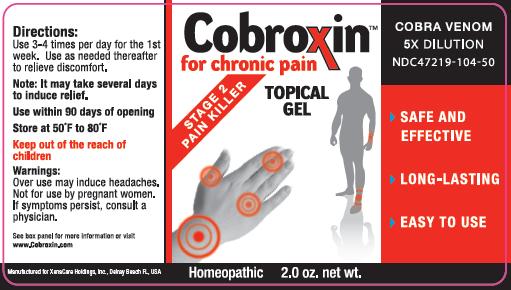
- Directions For Use:
-
Other Information:
- Do not use if container seal is broken prior to opening.
- This product is intended for use in cases of recurring joint pain.
- This product is NOT intended to treat disease. It provides a temporary level of comfort, relief, and a feeling of wellness.
- This product has been determined to be safe and effective for mild to moderate (Stage 2) chronic pain, as indicated by the Homeopathic Pharmacopeia of the United States.
- SPL UNCLASSIFIED SECTION
- Inactive Ingredients:
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COBROXIN FOR CHRONIC PAIN
naja naja venom gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47219-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM .020 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIPROPYLENE GLYCOL (UNII: E107L85C40) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 2-(DIETHYLAMINO)ETHANOL (UNII: S6DL4M053U) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47219-104-50 1 in 1 BOX 1 58 mL in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 08/25/2009 Labeler - RECEPTOPHARM INC (145377888) Establishment Name Address ID/FEI Business Operations RECEPTOPHARM INC 145377888 ANALYSIS, MANUFACTURE, API MANUFACTURE Establishment Name Address ID/FEI Business Operations LIQUID PACKAGING RESOURCES 018935165 MANUFACTURE