COBROXIN FOR CHRONIC PAIN- naja naja venom gel
RECEPTOPHARM INC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
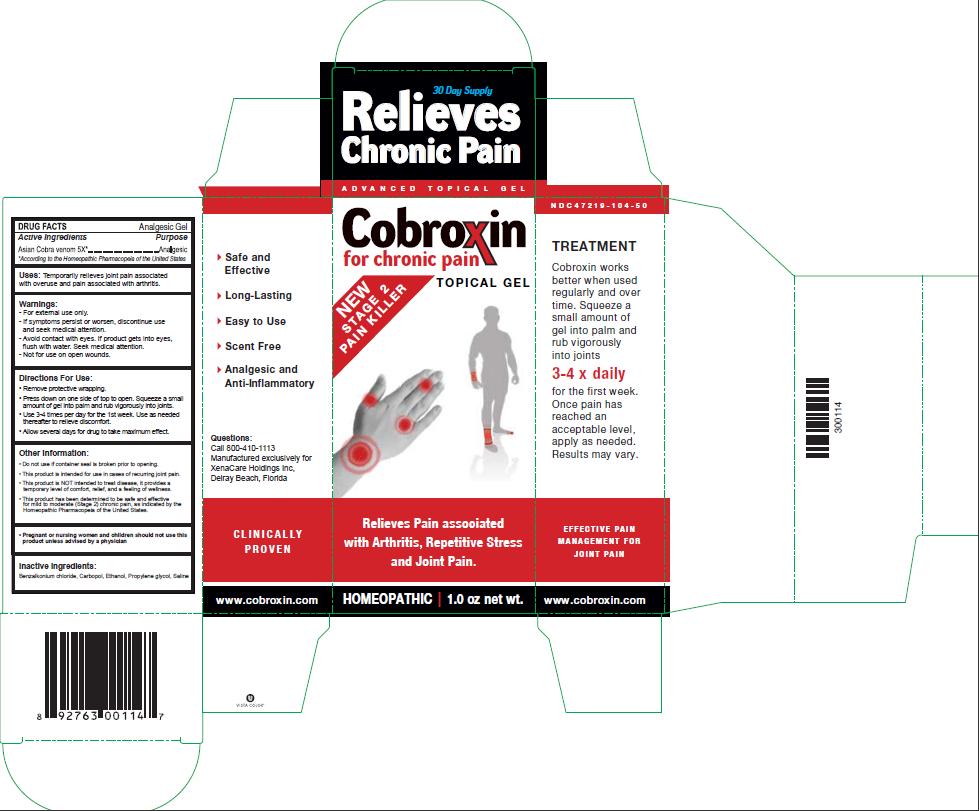
Uses: Temporarily relieves joint pain associated with overuse and pain associated with arthritis.
Warnings:
- -
- For external use only.
- -
- If symptoms persist or worsen, discontinue use and seek medical attention.
- -
- Avoid contact with eyes. If product gets into eyes, flush with water. Seek Medical attention.
- -
- Not for use on open wounds.
Directions For Use:
- Remove protective wrapping.
- Press down on one side of top to open. Squeeze a small amount of gel into palm and rub vigorously into joints.
- Use 3-4 times per day for the 1st week. Use as needed thereafter to relieve discomfort.
- Allow several days for drug to take maximum effect.
Other Information:
- Do not use if container seal is broken prior to opening.
- This product is intended for use in cases of recurring joint pain.
- This product is NOT intended to treat disease. It provides a temporary level of comfort, relief, and a feeling of wellness.
- This product has been determined to be safe and effective for mild to moderate (Stage 2) chronic pain, as indicated by the Homeopathic Pharmacopeia of the United States.
Pregnant or nursing women and children should not use this product unless advised by a physician
Inactive Ingredients:
Benzalkonium chloride, Methocel, Ethanol, Propylene glycol, Saline
Package Label - Principal Display Panel – 1.0 oz Gel Label
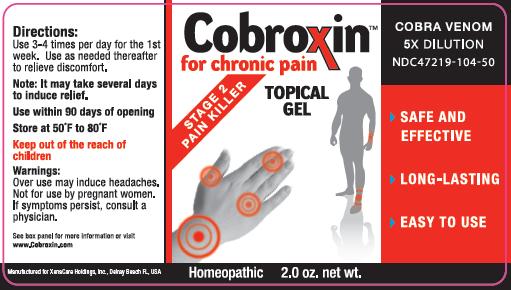
Package Label - Principal Display Panel – 1.0 oz Gel Carton