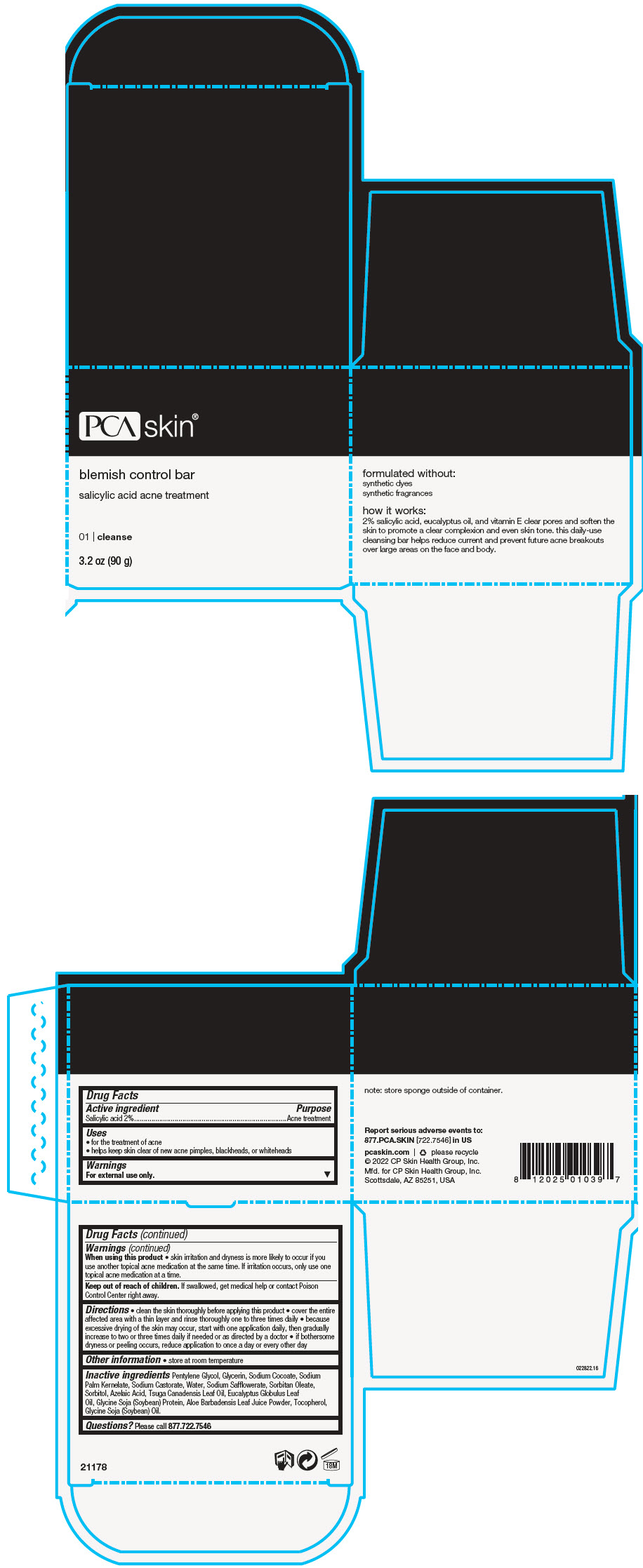
Label: BLEMISH CONTROL BAR- salicylic acid soap
- NDC Code(s): 68726-439-01
- Packager: CP Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other information
-
Inactive ingredients
Pentylene Glycol, Glycerin, Sodium Cocoate, Sodium Palm Kernelate, Sodium Castorate, Water, Sodium Safflowerate, Sorbitan Oleate, Sorbitol, Azelaic Acid, Tsuga Canadensis Leaf Oil, Eucalyptus Globulus Leaf Oil, Glycine Soja (Soybean) Protein, Aloe Barbadensis Leaf Juice Powder, Tocopherol, Glycine Soja (Soybean) Oil.
- Questions?
- PRINCIPAL DISPLAY PANEL - 90 g Jar Box
-
INGREDIENTS AND APPEARANCE
BLEMISH CONTROL BAR
salicylic acid soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68726-439 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength PENTYLENE GLYCOL (UNII: 50C1307PZG) GLYCERIN (UNII: PDC6A3C0OX) SODIUM COCOATE (UNII: R1TQH25F4I) SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) SODIUM CASTORATE (UNII: 6PAR8M5DCK) WATER (UNII: 059QF0KO0R) SODIUM SAFFLOWERATE (UNII: UHG9F87J7K) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) SORBITOL (UNII: 506T60A25R) AZELAIC ACID (UNII: F2VW3D43YT) TSUGA CANADENSIS LEAF OIL (UNII: Q1S51LGQ0G) EUCALYPTUS OIL (UNII: 2R04ONI662) SOY PROTEIN (UNII: R44IWB3RN5) ALOE VERA LEAF (UNII: ZY81Z83H0X) TOCOPHEROL (UNII: R0ZB2556P8) SOYBEAN OIL (UNII: 241ATL177A) Product Characteristics Color BROWN (Pale Beige) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68726-439-01 1 in 1 BOX 04/22/2016 1 90 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M006 04/22/2016 Labeler - CP Skin Health Group, Inc. (611921669)