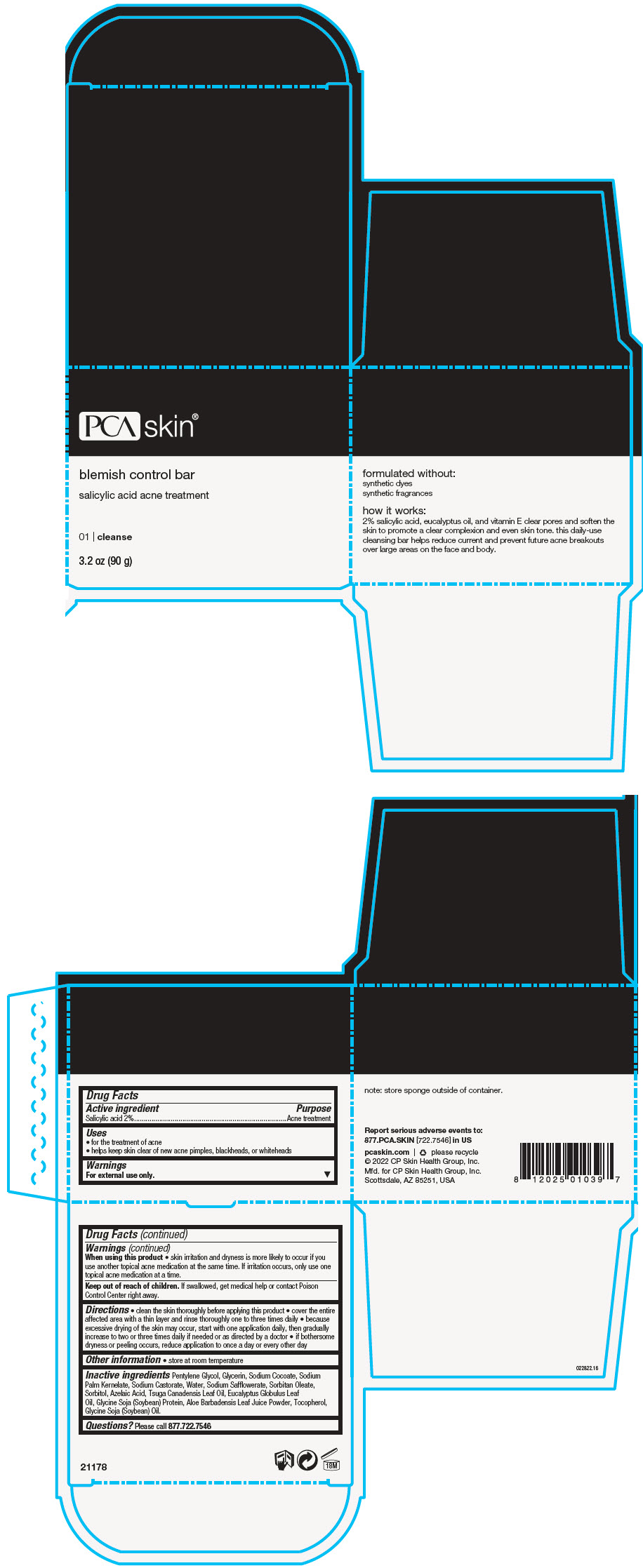
BLEMISH CONTROL BAR- salicylic acid soap
CP Skin Health Group, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic acid 2%
Uses
- for the treatment of acne
- helps keep skin clear of new acne pimples, blackheads, or whiteheads
Warnings
For external use only.
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Keep out of reach of children. If swallowed, get medical help or contact Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Other information
- store at room temperature
Inactive ingredients
Pentylene Glycol, Glycerin, Sodium Cocoate, Sodium Palm Kernelate, Sodium Castorate, Water, Sodium Safflowerate, Sorbitan Oleate, Sorbitol, Azelaic Acid, Tsuga Canadensis Leaf Oil, Eucalyptus Globulus Leaf Oil, Glycine Soja (Soybean) Protein, Aloe Barbadensis Leaf Juice Powder, Tocopherol, Glycine Soja (Soybean) Oil.
Questions?
Please call 877.722.7546
PRINCIPAL DISPLAY PANEL - 90 g Jar Box
PCA skin®
blemish control bar
salicylic acid acne treatment
01 | cleanse
3.2 oz (90 g)
CP Skin Health Group, Inc.