Label: ARIPIPRAZOLE tablet, orally disintegrating
-
NDC Code(s):
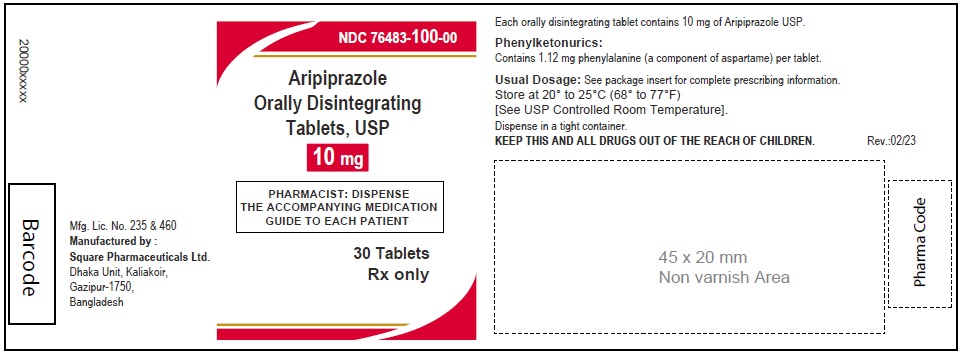
76483-100-00,
76483-100-01,
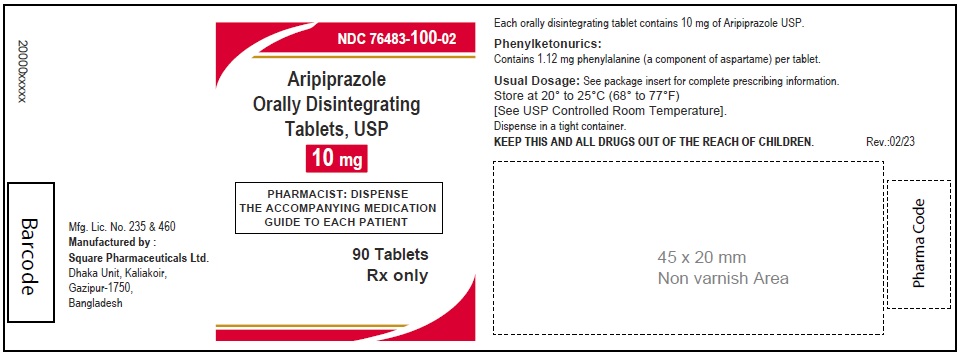
76483-100-02,
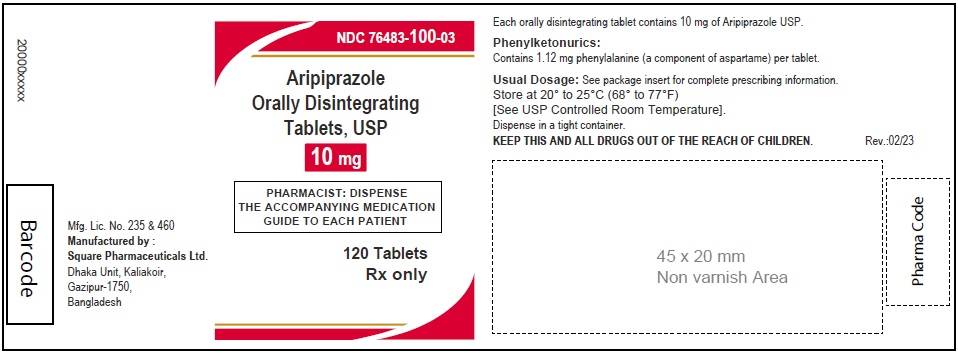
76483-100-03, view more76483-100-04, 76483-101-00, 76483-101-01, 76483-101-02, 76483-101-03, 76483-101-04, 76483-102-00, 76483-102-01, 76483-102-02, 76483-102-03, 76483-102-04, 76483-103-00, 76483-103-01, 76483-103-02, 76483-103-03, 76483-103-04
- Packager: SQUARE PHARMACEUTICALS LIMITED
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
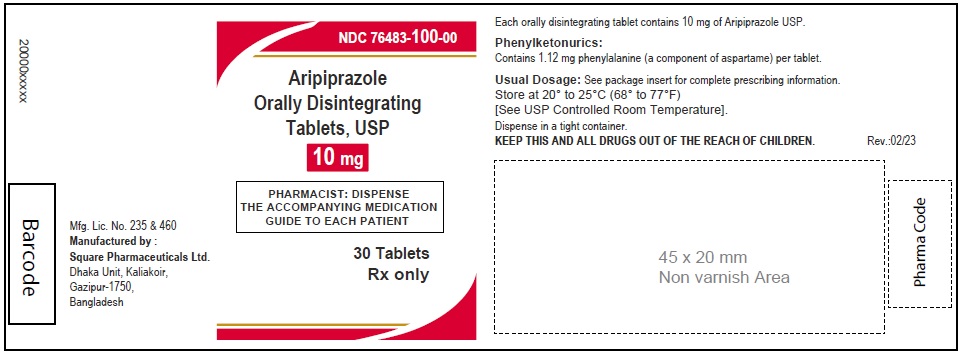
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
30 Tablets
Rx Only
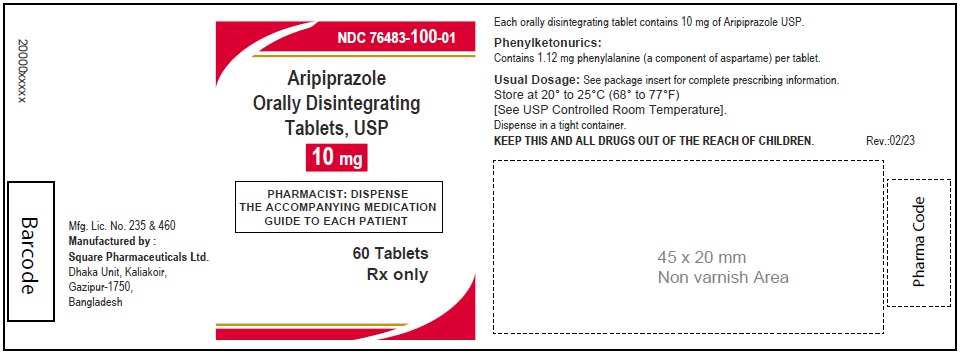
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
60 Tablets
Rx Only
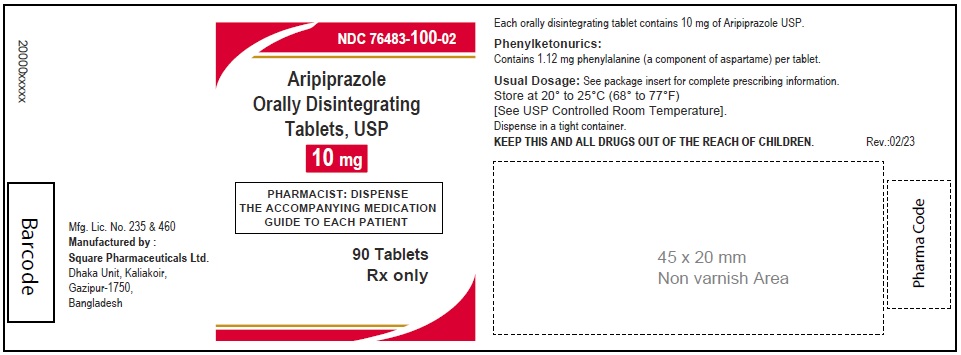
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
90 Tablets
Rx Only
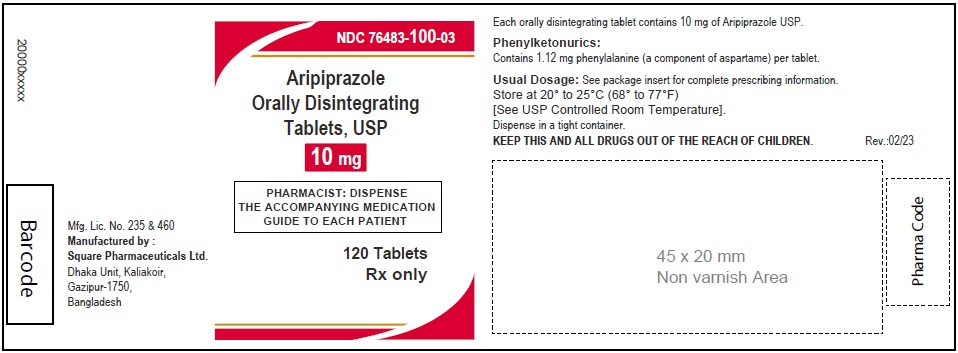
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
120 Tablets
Rx Only
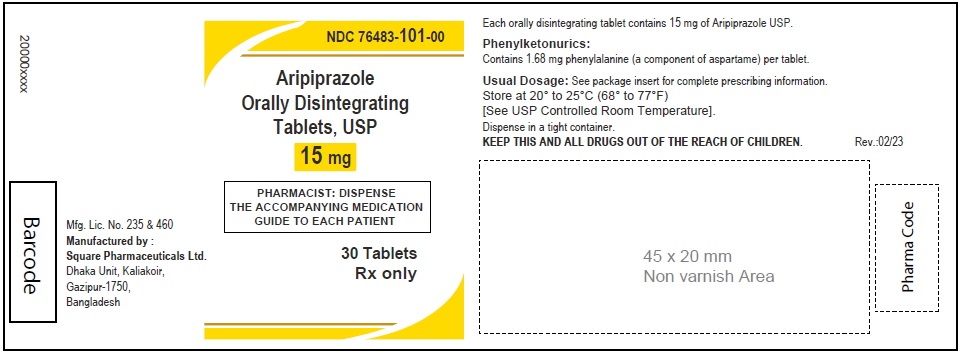
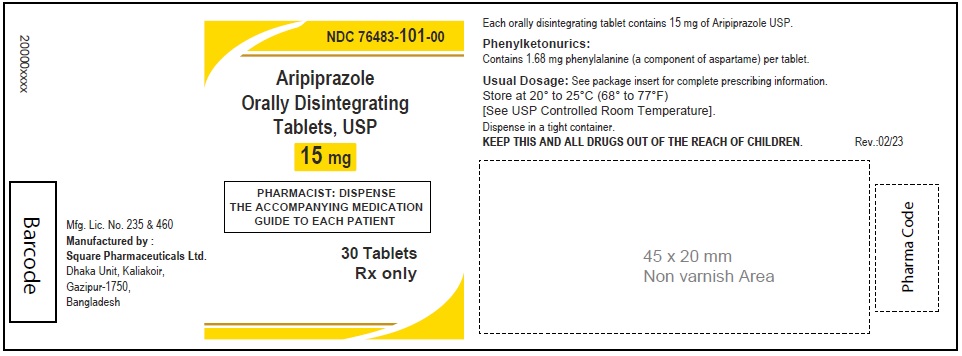
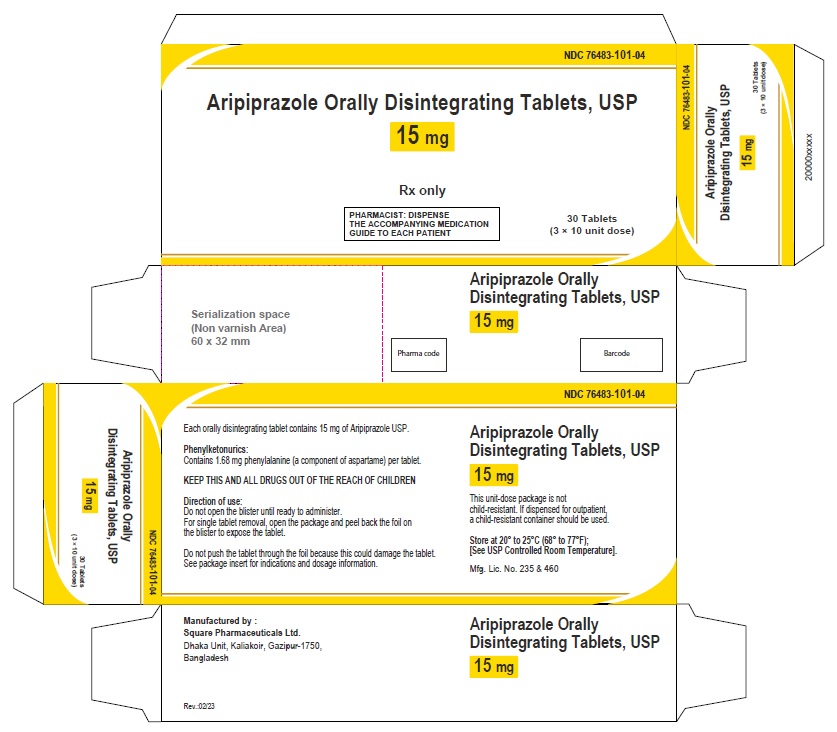
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
30 Tablets
Rx Only
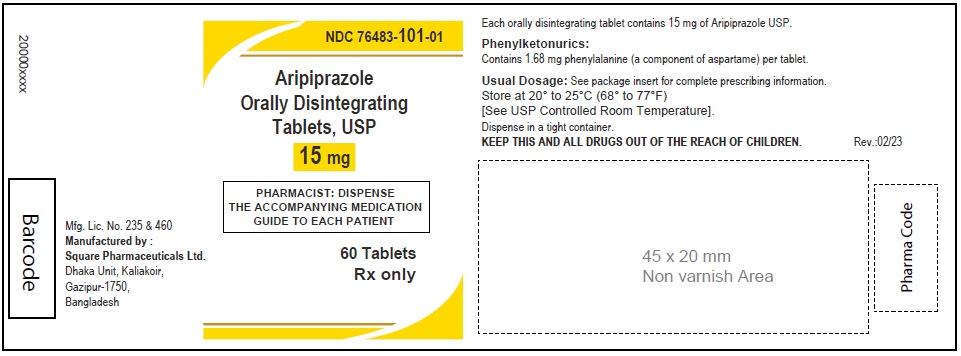
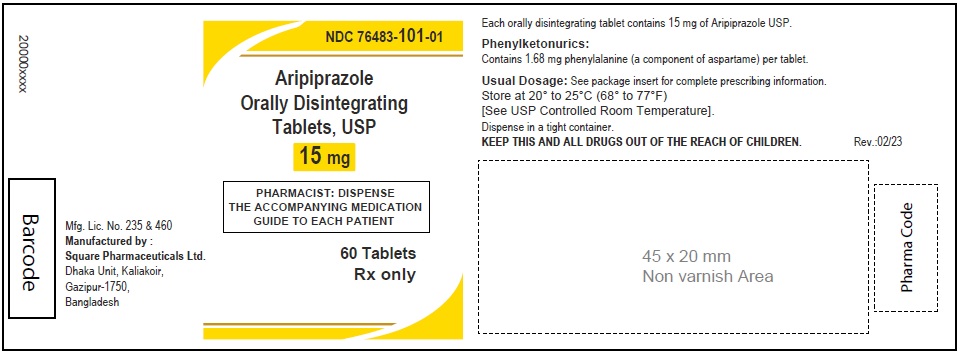
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
60 Tablets
Rx Only
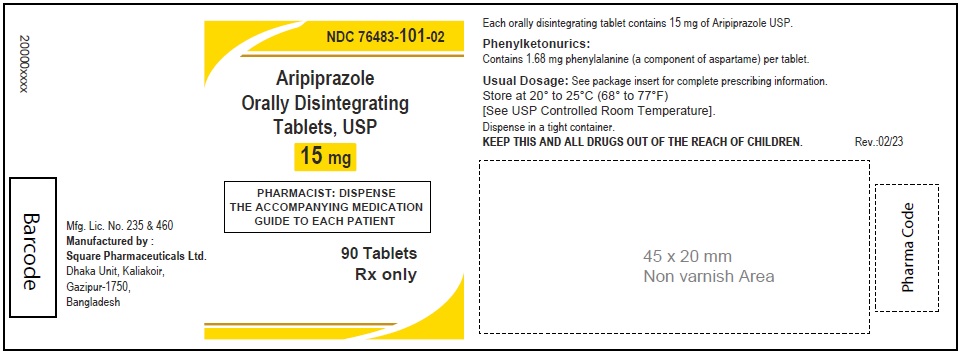
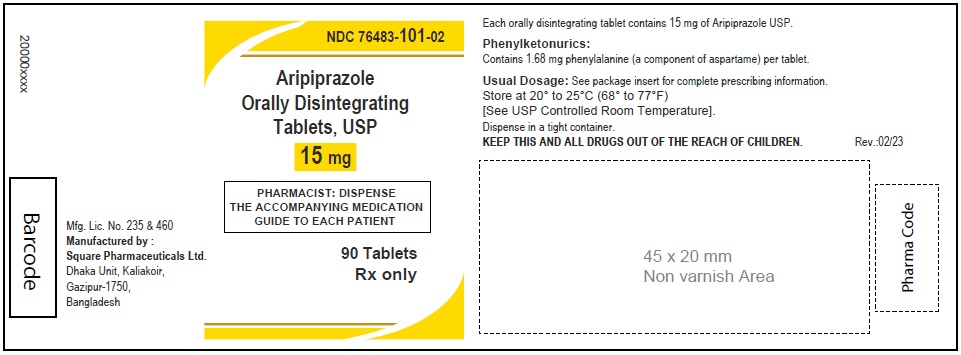
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
90 Tablets
Rx Only
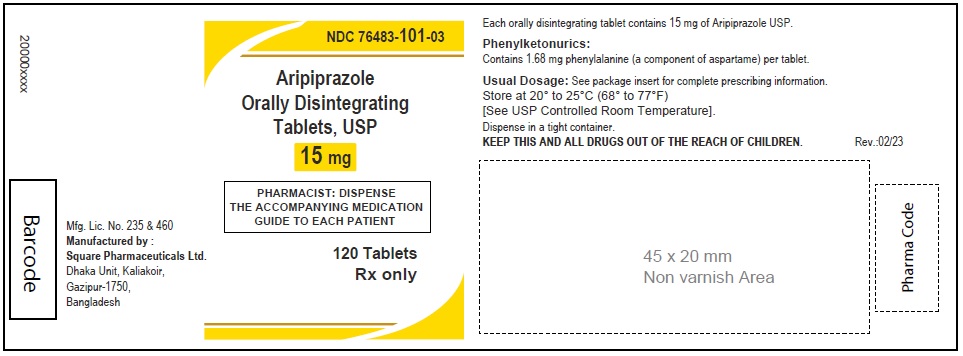
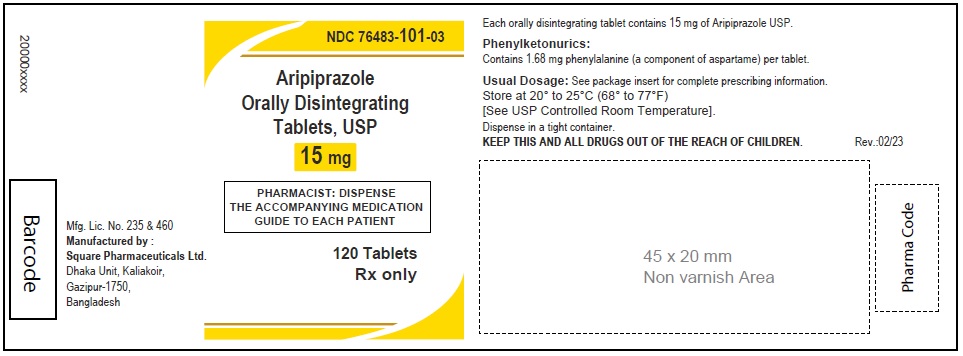
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
120 Tablets
Rx Only
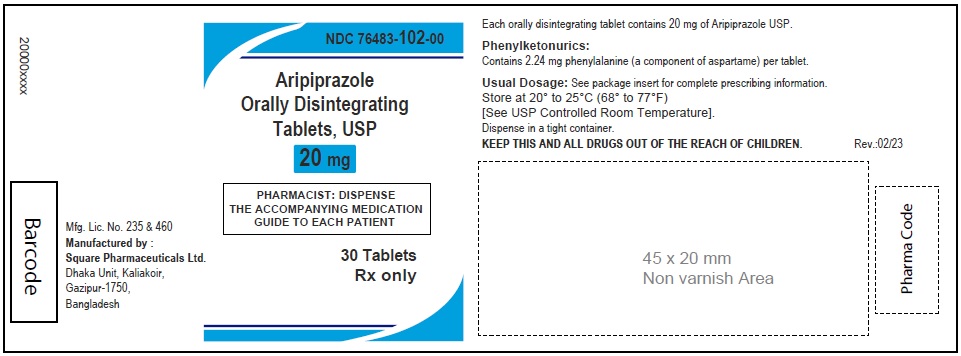
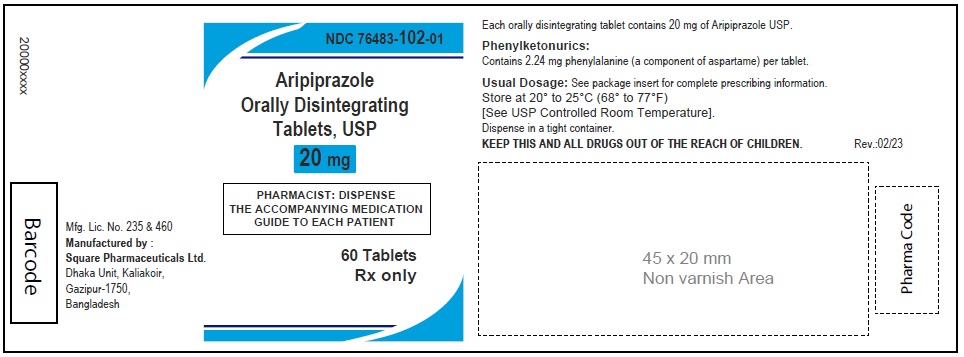
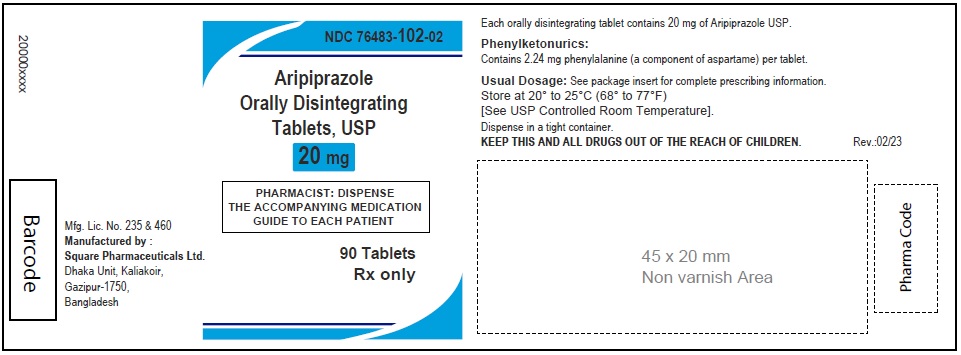
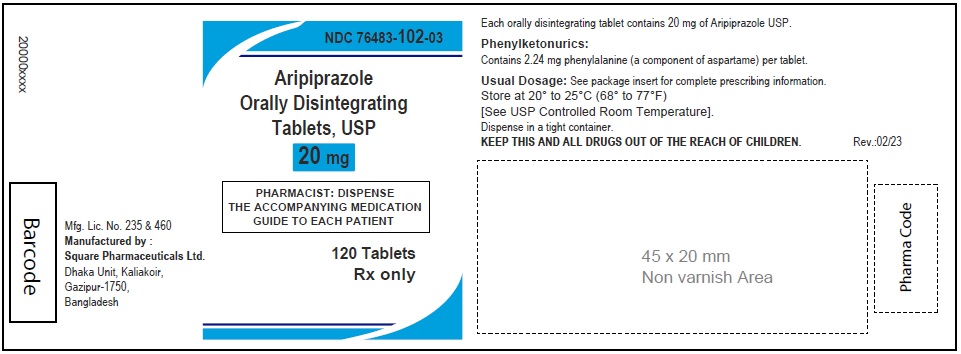
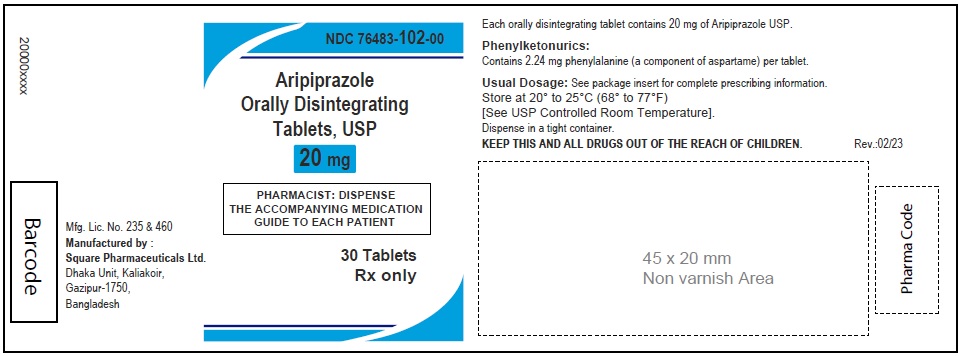
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
30 Tablets
Rx Only
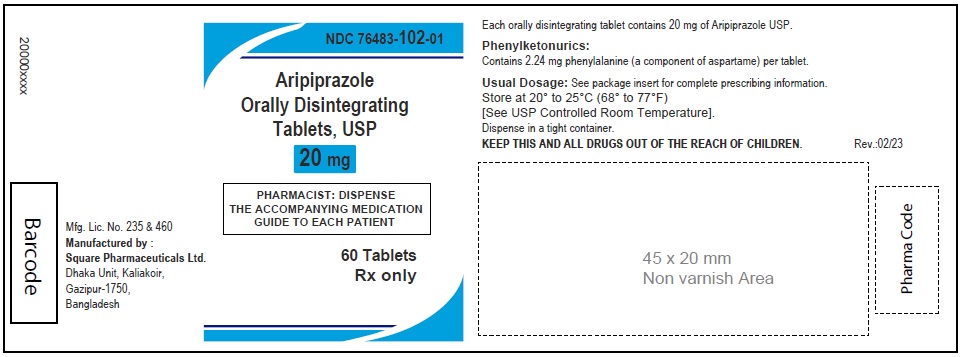
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
60 Tablets
Rx Only
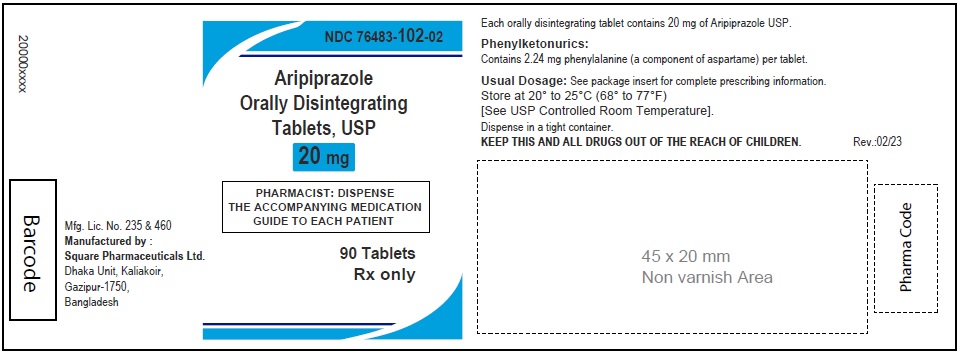
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
90 Tablets
Rx Only
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
120 Tablets
Rx Only
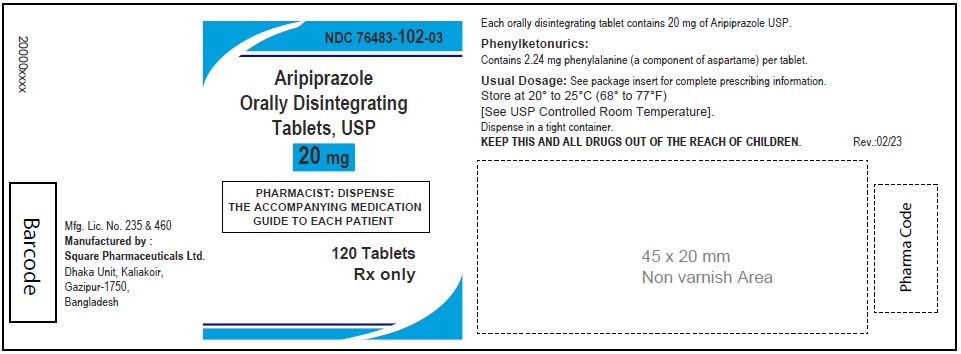
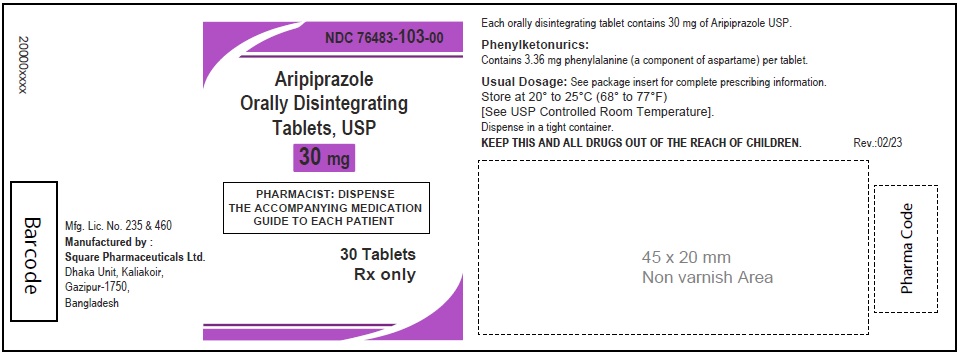
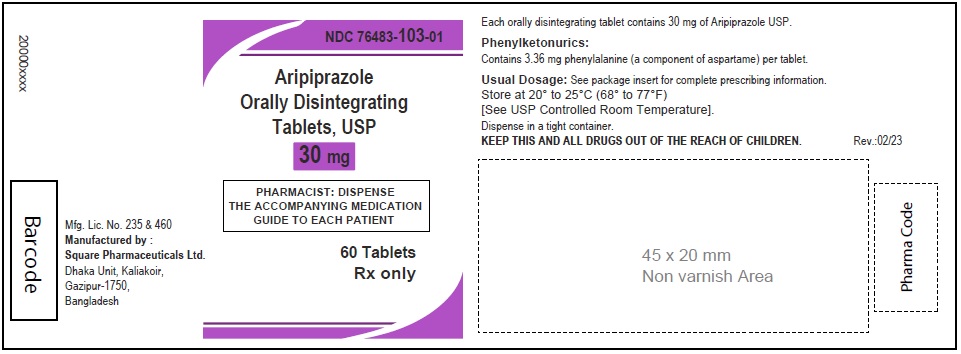
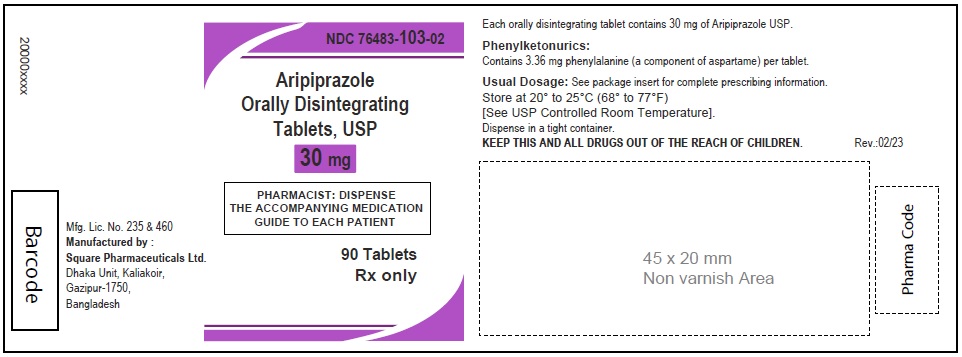
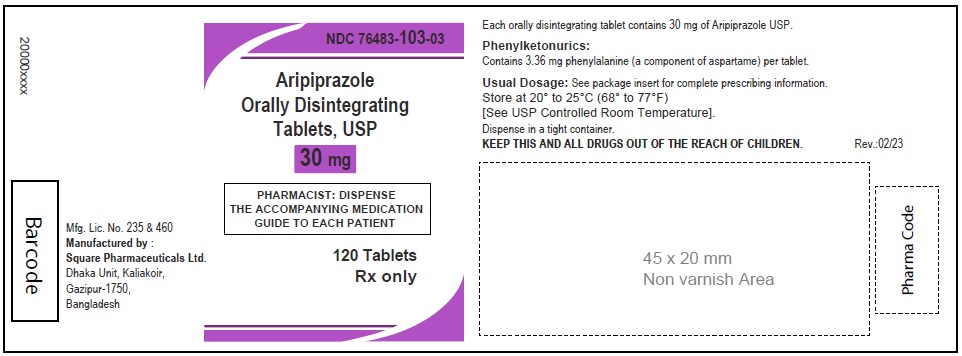
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
30 Tablets
Rx Only
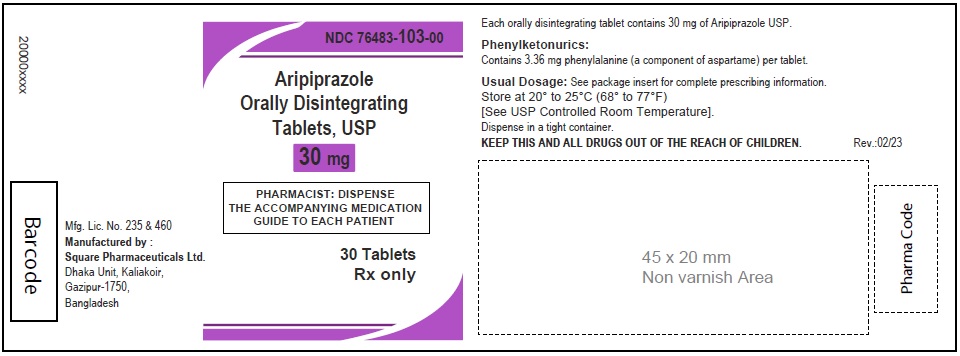
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
60 Tablets
Rx Only
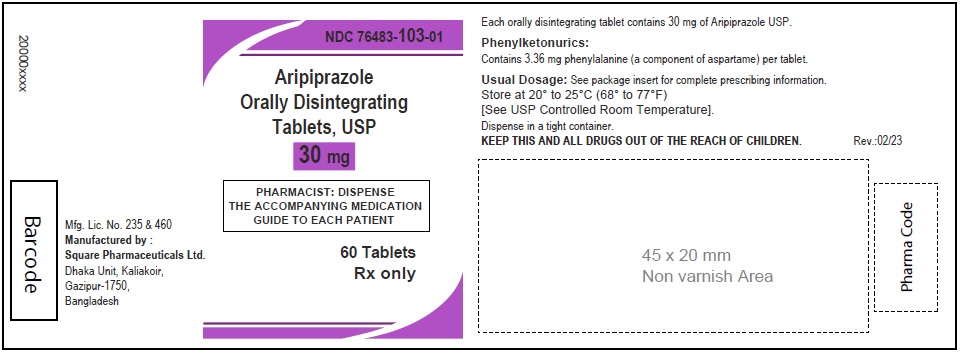
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
90 Tablets
Rx Only
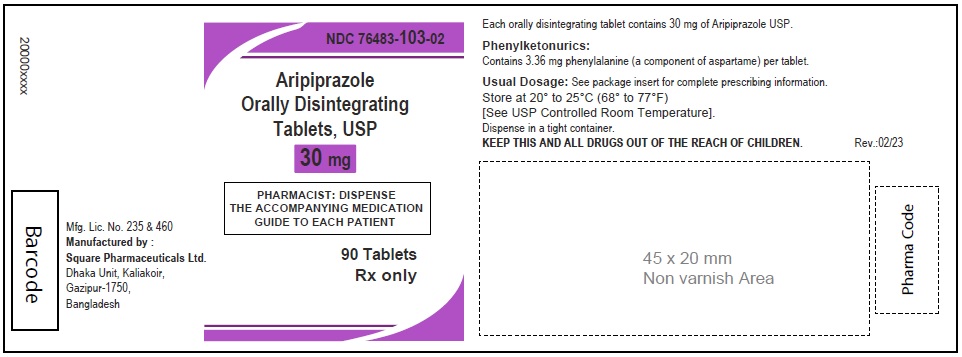
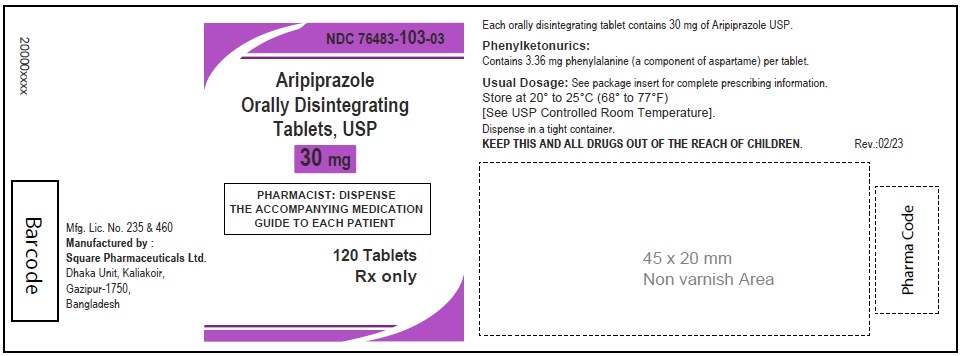
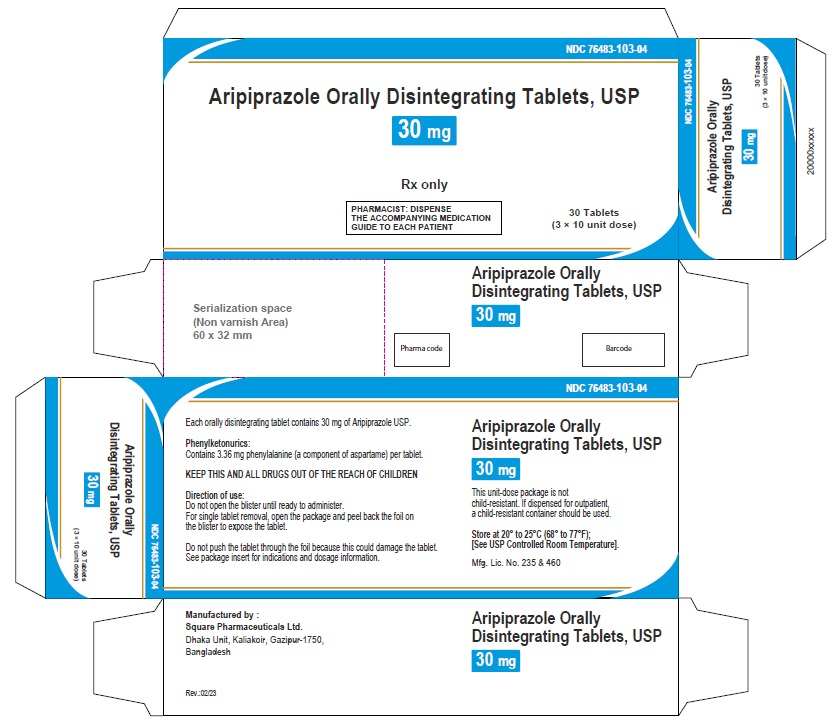
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
120 Tablets
Rx Only
Aripiprazole Orally Disintegrating Tablets 10 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 10 mg aripiprazole USP
NDC 76483-100-04
Rx Only

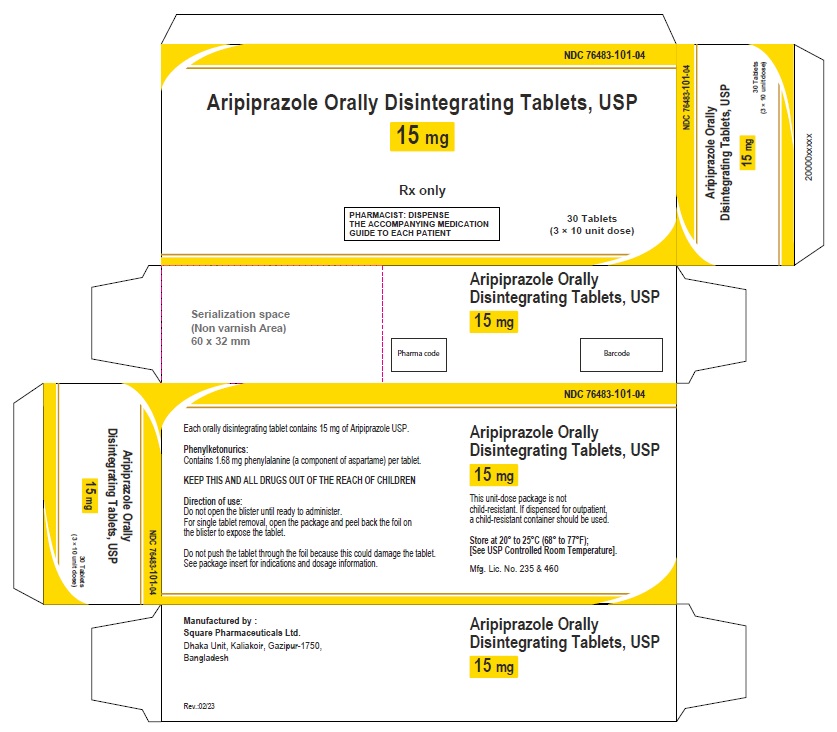
Aripiprazole Orally Disintegrating Tablets 15 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 15 mg aripiprazole USP
NDC 76483-101-04
Rx Only
Aripiprazole Orally Disintegrating Tablets, 20 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 20 mg aripiprazole USP
NDC 76483-102-04
Rx Only

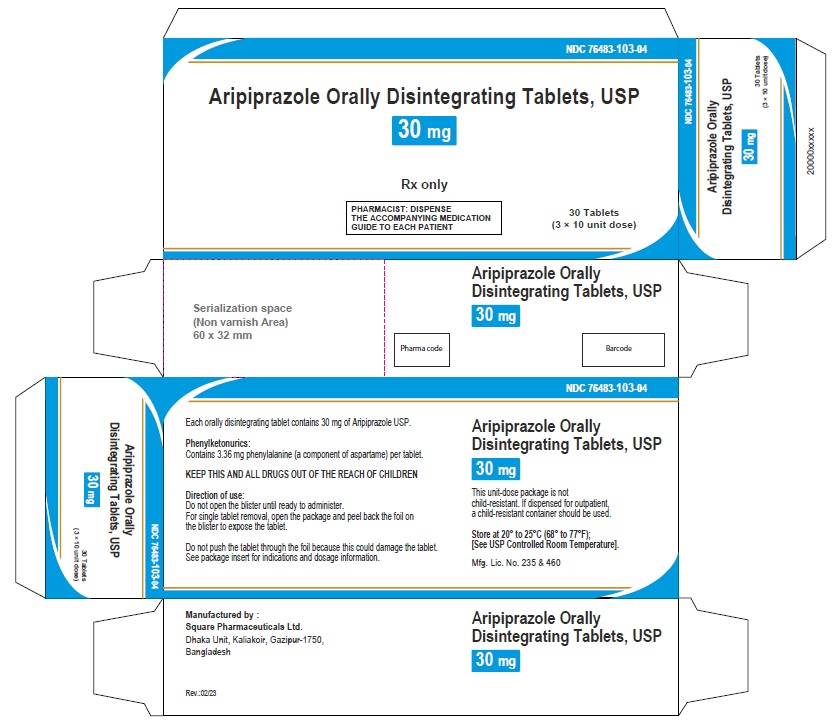
Aripiprazole Orally Disintegrating Tablets 30 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 30 mg aripiprazole USP
NDC 76483-103-04
Rx Only
-
INGREDIENTS AND APPEARANCE
ARIPIPRAZOLE
aripiprazole tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76483-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 10 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape CAPSULE Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZF;41 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76483-100-00 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 2 NDC:76483-100-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 3 NDC:76483-100-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 4 NDC:76483-100-03 120 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 5 NDC:76483-100-04 30 in 1 CARTON; Type 0: Not a Combination Product 02/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090165 07/20/2022 ARIPIPRAZOLE
aripiprazole tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76483-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape ROUND Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZF;42 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76483-101-00 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 2 NDC:76483-101-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 3 NDC:76483-101-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 4 NDC:76483-101-03 120 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 5 NDC:76483-101-04 30 in 1 CARTON; Type 0: Not a Combination Product 02/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090165 07/20/2022 ARIPIPRAZOLE
aripiprazole tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76483-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) MANNITOL (UNII: 3OWL53L36A) PEPPERMINT (UNII: V95R5KMY2B) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape OCTAGON (8 SIDED) (BARREL) Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZF;43 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76483-102-00 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 2 NDC:76483-102-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 3 NDC:76483-102-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 4 NDC:76483-102-03 120 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 5 NDC:76483-102-04 30 in 1 CARTON; Type 0: Not a Combination Product 02/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090165 07/20/2022 ARIPIPRAZOLE
aripiprazole tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76483-103 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 30 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) MANNITOL (UNII: 3OWL53L36A) PEPPERMINT (UNII: V95R5KMY2B) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape ROUND (ROUND) Size 10mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZF;44 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76483-103-00 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 2 NDC:76483-103-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 3 NDC:76483-103-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 4 NDC:76483-103-03 120 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 5 NDC:76483-103-04 30 in 1 CARTON; Type 0: Not a Combination Product 02/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090165 07/20/2022 Labeler - SQUARE PHARMACEUTICALS LIMITED (731487153) Registrant - SQUARE PHARMACEUTICALS LIMITED (731487153) Establishment Name Address ID/FEI Business Operations SQUARE PHARMACEUTICALS LIMITED, Dhaka unit 850366520 ANALYSIS(76483-100, 76483-101, 76483-102, 76483-103) , LABEL(76483-100, 76483-101, 76483-102, 76483-103) , MANUFACTURE(76483-100, 76483-101, 76483-102, 76483-103) , PACK(76483-100, 76483-101, 76483-102, 76483-103)