PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
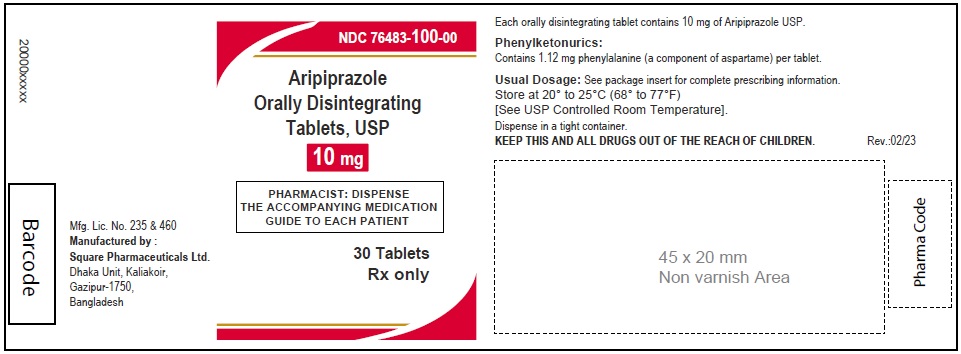
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
30 Tablets
Rx Only
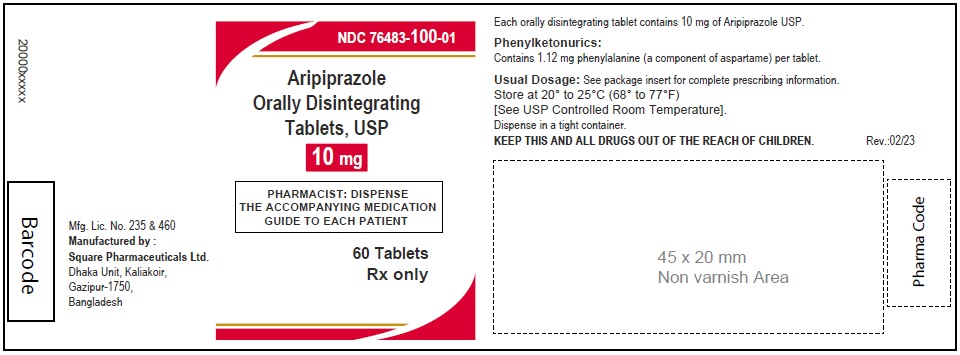
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
60 Tablets
Rx Only
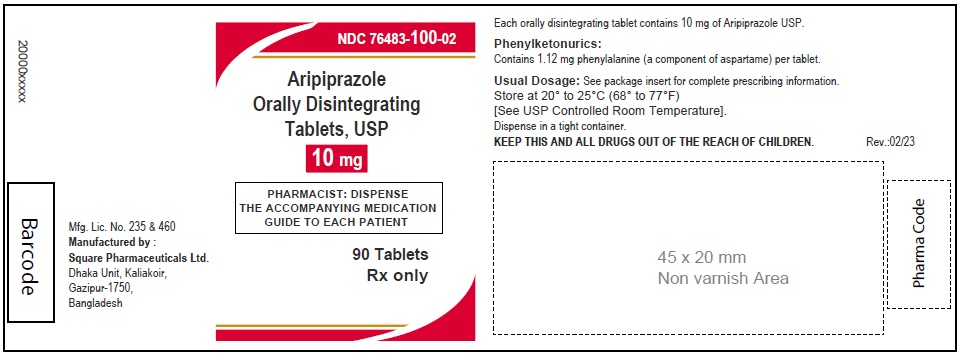
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
90 Tablets
Rx Only
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
120 Tablets
Rx Only
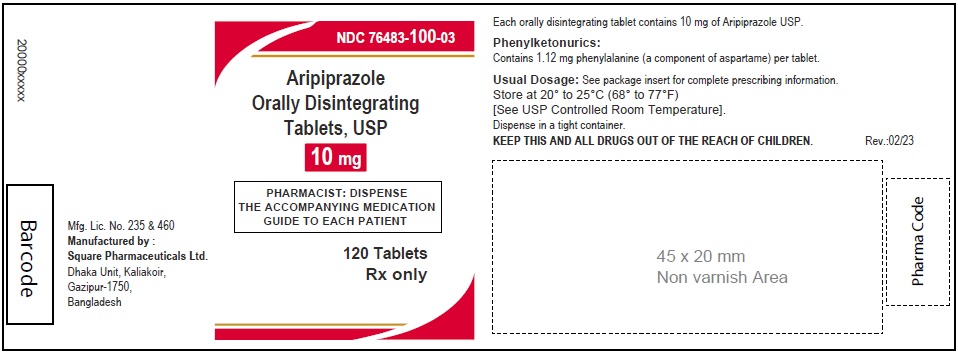
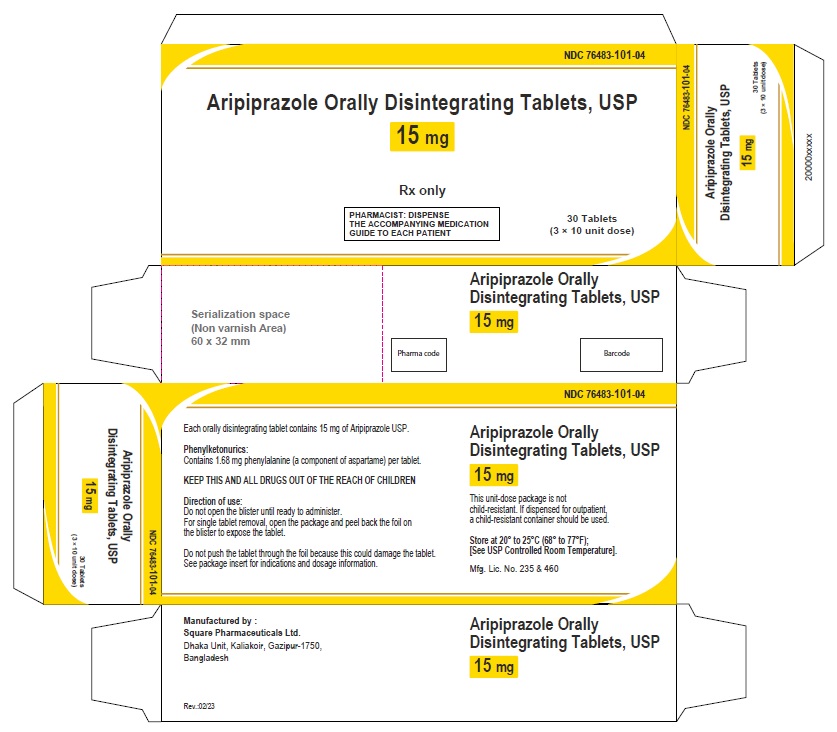
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
30 Tablets
Rx Only
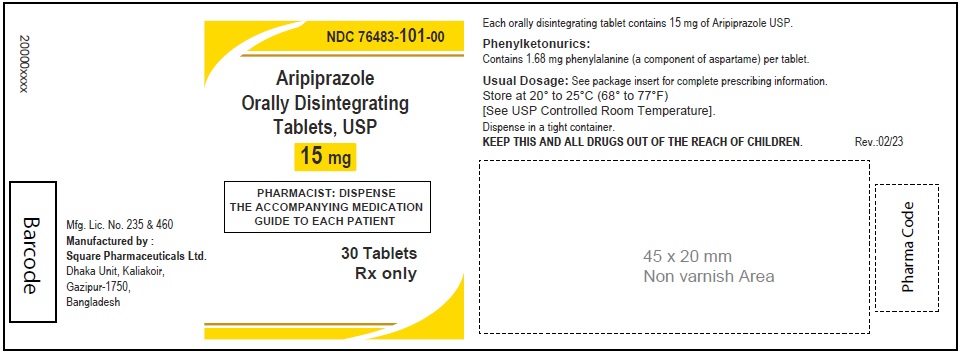
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
60 Tablets
Rx Only
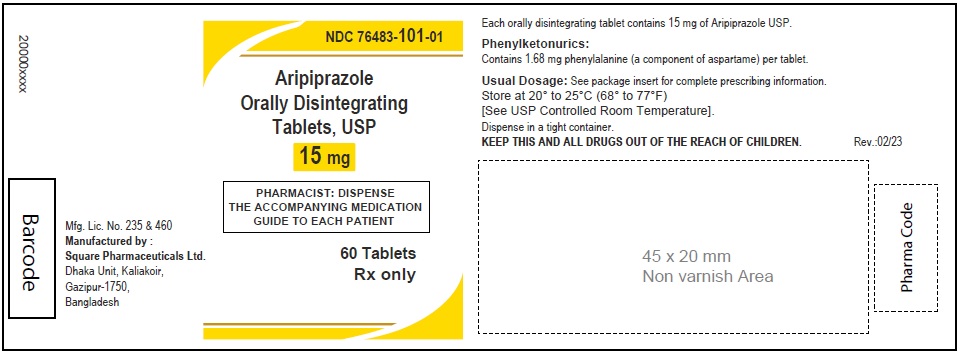
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
90 Tablets
Rx Only
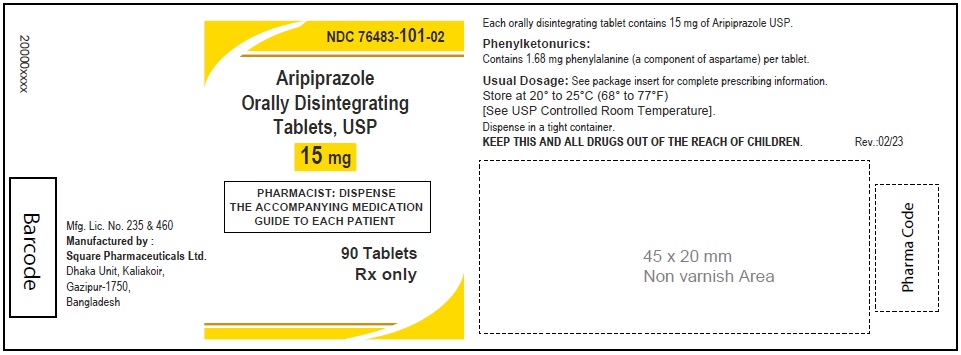
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
120 Tablets
Rx Only
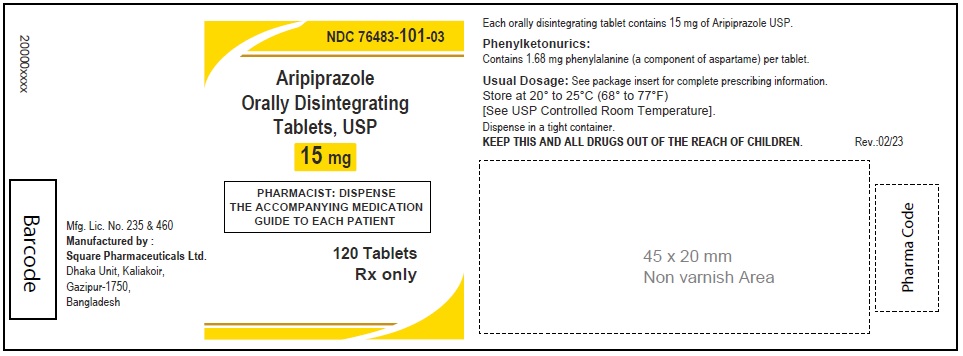
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
30 Tablets
Rx Only
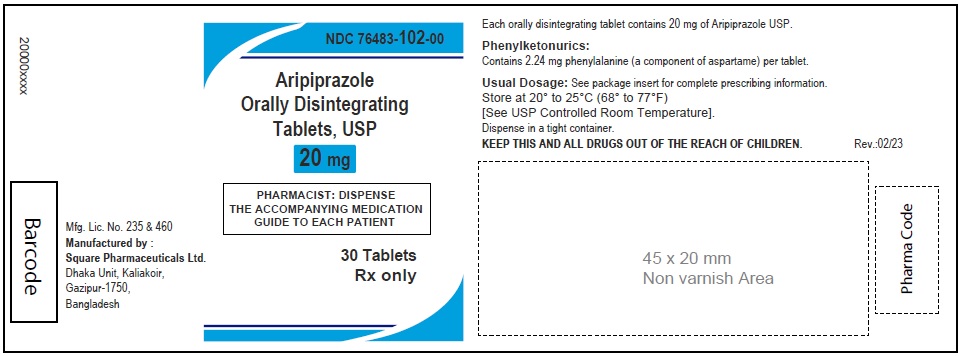
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
60 Tablets
Rx Only
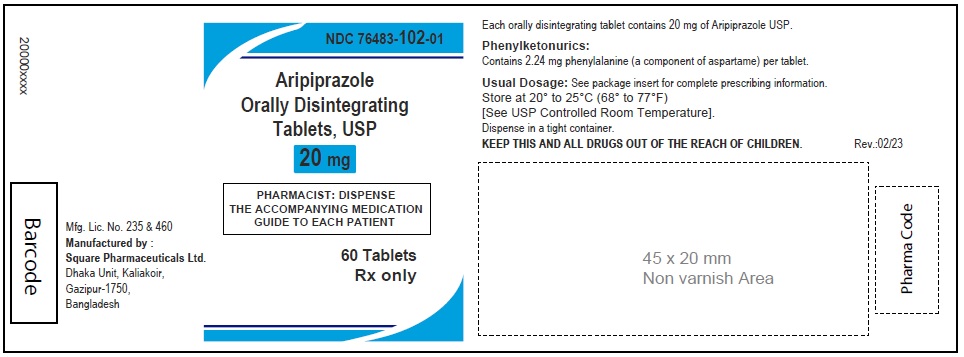
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
90 Tablets
Rx Only
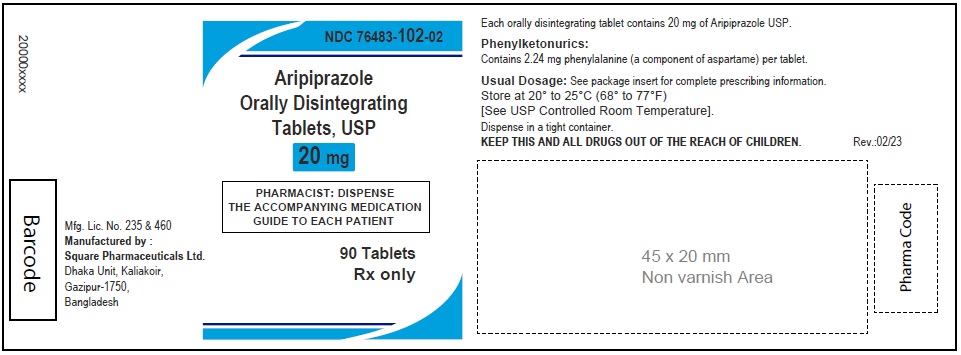
Aripiprazole Orally Disintegrating Tablets, USP 20 mg
120 Tablets
Rx Only
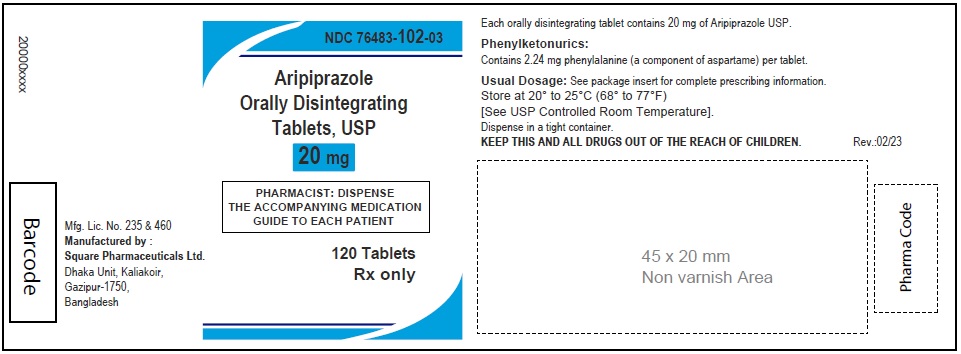
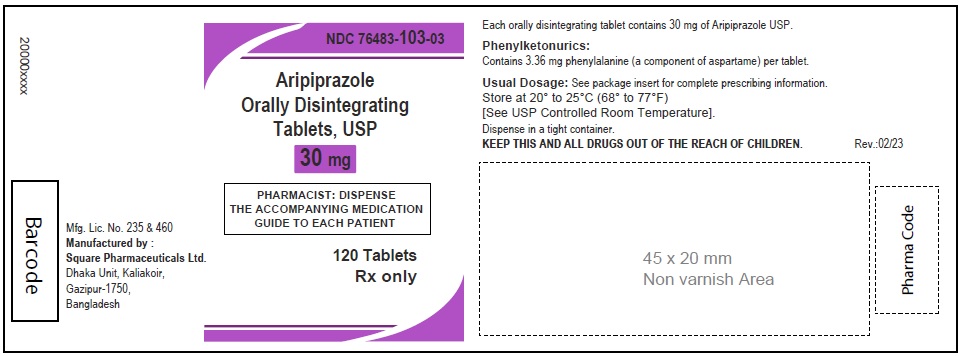
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
30 Tablets
Rx Only
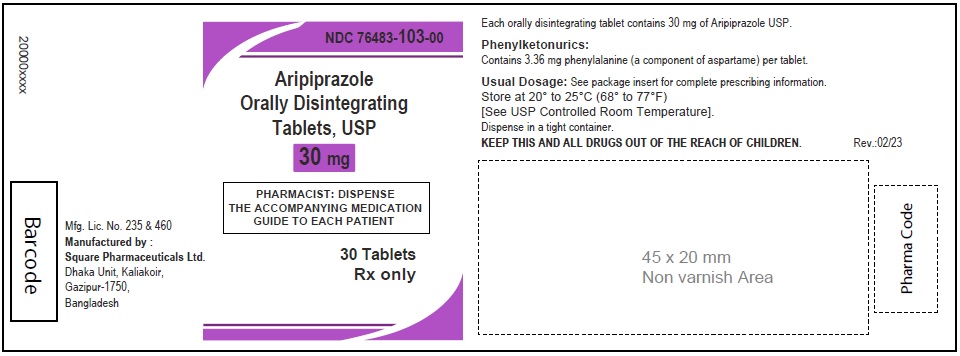
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
60 Tablets
Rx Only
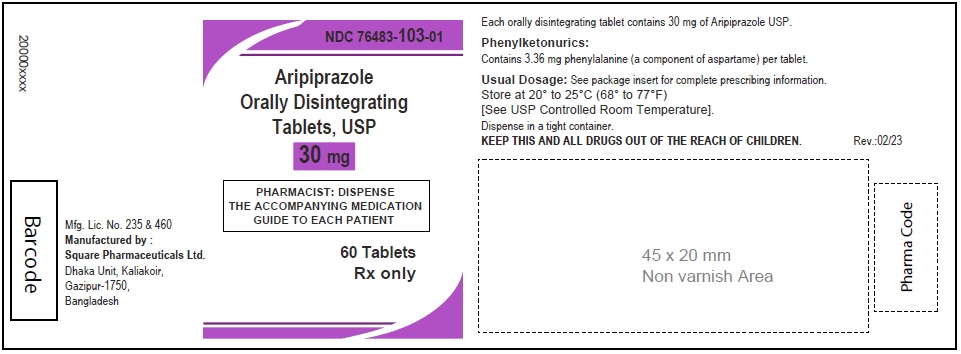
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
90 Tablets
Rx Only
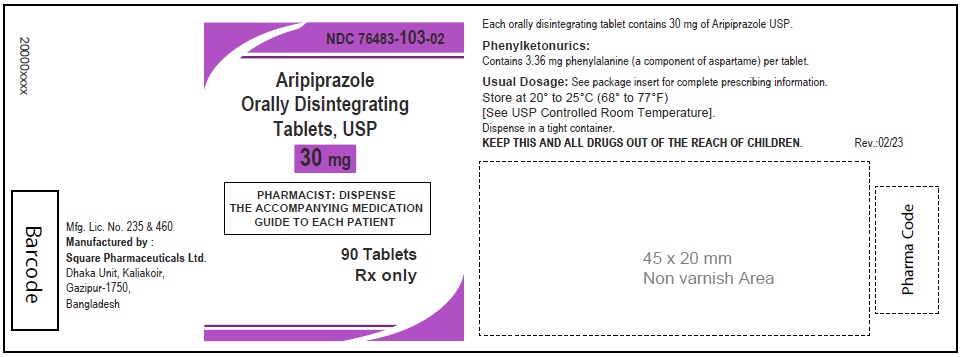
Aripiprazole Orally Disintegrating Tablets, USP 30 mg
120 Tablets
Rx Only
Aripiprazole Orally Disintegrating Tablets 10 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 10 mg aripiprazole USP
NDC 76483-100-04
Rx Only

Aripiprazole Orally Disintegrating Tablets 15 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 15 mg aripiprazole USP
NDC 76483-101-04
Rx Only
Aripiprazole Orally Disintegrating Tablets, 20 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 20 mg aripiprazole USP
NDC 76483-102-04
Rx Only

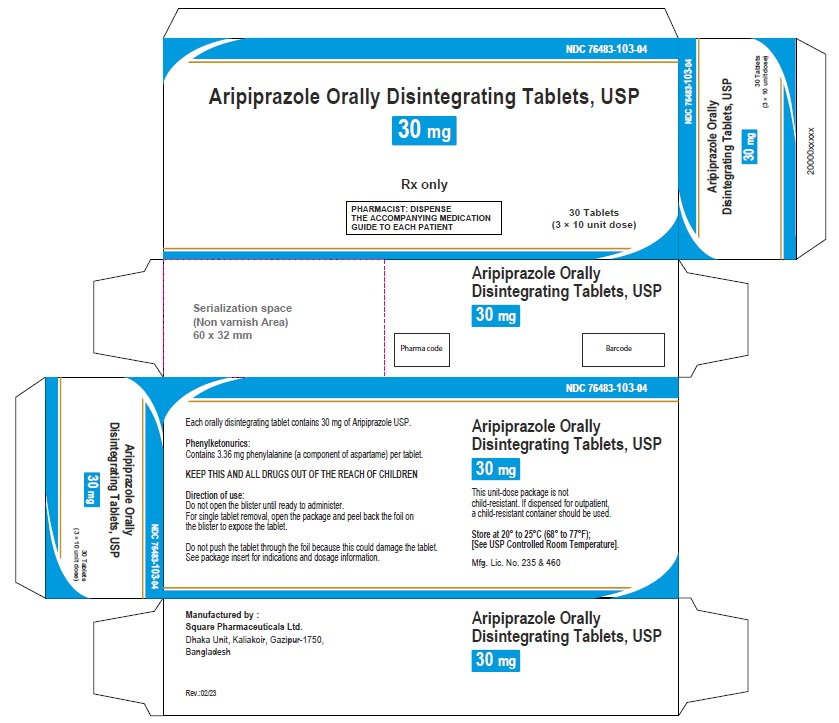
Aripiprazole Orally Disintegrating Tablets 30 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 30 mg aripiprazole USP
NDC 76483-103-04
Rx Only