Label: MLK F1 KIT- marcaine, lidocaine, kenalog, povidone iodine kit
-
NDC Code(s):
0409-1162-18,
55150-164-02,
67777-419-02,
70121-1049-5, view more76420-740-01
- Packager: Asclemed USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BUPIVACAINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for BUPIVACAINE HYDROCHLORIDE INJECTION.
BUPIVACAINE HYDROCHLORIDE injection, for infiltration, perineural, caudal, epidural, or retrobulbar use
Initial U.S. Approval: 1972WARNING: RISK OF CARDIAC ARREST WITH USE OF BUPIVACAINE HYDROCHLORIDE INJECTION IN OBSTETRICAL ANESTHESIA
See full prescribing information for complete boxed warning.
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride Injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride Injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary ( 5.1).
INDICATIONS AND USAGE
Bupivacaine Hydrochloride Injection contains bupivacaine, an amide local anesthetic. Bupivacaine Hydrochloride Injection is indicated in adults for the production of local or regional anesthesia or analgesia for surgery, dental and oral surgery procedures, diagnostic and therapeutic procedures, and for obstetrical procedures. For each type of block indicated to produce local or regional anesthesia or analgesia, specific concentrations and presentations are recommended. ( 1, 2.2)
Limitations of Use
Not all blocks are indicated for use with Bupivacaine Hydrochloride Injection given clinically significant risks associated with use. ( 1, 2.2, 4, 5.1, 5.5, 5.7, 5.9)
DOSAGE AND ADMINISTRATION
- Not for intrathecal use. ( 2.1)
- Avoid use of solutions containing antimicrobial preservatives (i.e., multiple-dose vials) for epidural or caudal anesthesia. ( 2.1)
- See full prescribing information for:
- Recommended concentrations and dosages of Bupivacaine Hydrochloride Injection according to type of block. ( 2.2)
- Additional dosage and administration information pertaining to use in epidural anesthesia and use in ophthalmic surgery. ( 2.3, 2.6)
DOSAGE FORMS AND STRENGTHS
Bupivacaine Hydrochloride Injection, USP are available in following concentrations. See full prescribing information for detailed description of each formulation. ( 3)
CONTRAINDICATIONS
- Obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death. ( 4)
- Intravenous regional anesthesia (Bier Block). ( 4)
- Known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride Injection. ( 4)
WARNINGS AND PRECAUTIONS
- Dose-Related Toxicity: Monitor cardiovascular and respiratory vital signs and patient's state of consciousness after injection of Bupivacaine Hydrochloride Injection. ( 5.2)
- Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. See full prescribing information for more detail on managing these risks. ( 5.3)
- Chondrolysis with Intra-Articular Infusion: Intra-articular infusions of local anesthetics including Bupivacaine Hydrochloride Injection following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. ( 5.5)
- Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block): There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). ( 5.7)
- Risk of Systemic Toxicities with Unintended Intravascular or Intrathecal Injection: Unintended intravascular or intrathecal injection may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Aspirate for blood or cerebrospinal fluid (where applicable) prior to each dose. ( 5.9)
ADVERSE REACTIONS
Most common adverse reactions are related to the central nervous system and the cardiovascular system. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Local Anesthetics: The toxic effects of local anesthetics are additive. Monitor for neurologic and cardiovascular effects when additional local anesthetics are administered. ( 7.1)
- Drugs Associated with Methemoglobinemia: Patients are at increased risk of developing methemoglobinemia when concurrently exposed to nitrates, nitrites, local anesthetics, antineoplastic agents, antibiotics, antimalarials, anticonvulsants, and other drugs. ( 7.5)
- Potent Inhalation Anesthetics: Serious dose-related cardiac arrhythmias may occur if preparations containing a vasoconstrictor such as epinephrine are used in patients during or following the administration of potent inhalation anesthetics. ( 7.6)
USE IN SPECIFIC POPULATIONS
- Pediatric Use: Administration of Bupivacaine Hydrochloride Injection in pediatric patients younger than 12 years is not recommended. ( 8.4)
- Geriatric Use: Patients 65 years and over, particularly those with hypertension, may be at increased risk for developing hypotension while undergoing anesthesia with Bupivacaine Hydrochloride Injection. ( 8.5)
- Moderate to Severe Hepatic Impairment: Consider increased monitoring for bupivacaine systemic toxicity. ( 8.6)
See 17 for PATIENT COUNSELING INFORMATION and PATIENT COUNSELING INFORMATION.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF CARDIAC ARREST WITH USE OF BUPIVACAINE HYDROCHLORIDE INJECTION IN OBSTETRICAL ANESTHESIA
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
2.2 Recommended Concentrations and Dosages of Bupivacaine Hydrochloride Injection
2.3 Use in Epidural Anesthesia
2.6 Use in Ophthalmic Surgery
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Cardiac Arrest with Use of Bupivacaine Hydrochloride Injection in Obstetrical Anesthesia
5.2 Dose-Related Toxicity
5.3 Methemoglobinemia
5.5 Chondrolysis with Intra-Articular Infusion
5.7 Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block)
5.9 Risk of Systemic Toxicities with Unintended Intravascular or Intrathecal Injection
5.10 Risk of Toxicity in Patients with Hepatic Impairment
5.11 Risk of Use in Patients with Impaired Cardiovascular Function
5.14 Risk of Adverse Reactions with Use in Head and Neck Area
5.15 Risk of Respiratory Arrest with Use in Ophthalmic Surgery
5.16 Risk of Inadvertent Trauma to Tongue, Lips, and Buccal Mucosa in Dental Applications
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Local Anesthetics
7.5 Drugs Associated with Methemoglobinemia
7.6 Potent Inhalation Anesthetics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Mechanism of Action
Hemodynamics
Pharmacokinetics and Metabolism
General
Use in the Head and Neck Area
Information for Patients
Clinically Significant Drug Interactions
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Labor and Delivery
Nursing Mothers
Pediatric Use
Systemic
Management of Local Anesthetic Emergencies
Epidural Anesthesia
Caudal and Lumbar Epidural Block
Adults
Children
General
Vaccination
Neurologic
General
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Nursing Mothers
Pediatric Use
Geriatric Use
Dosage
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF CARDIAC ARREST WITH USE OF BUPIVACAINE HYDROCHLORIDE INJECTION IN OBSTETRICAL ANESTHESIA
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride Injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride Injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
Bupivacaine Hydrochloride Injection is indicated in adults for the production of local or regional anesthesia or analgesia for surgery, dental and oral surgery procedures, diagnostic and therapeutic procedures, and for obstetrical procedures. Specific concentrations and presentations of Bupivacaine Hydrochloride Injection are recommended for each type of block indicated to produce local or regional anesthesia or analgesia [see Dosage and Administration (2.2)].
Limitations of Use
Not all blocks are indicated for use with Bupivacaine Hydrochloride Injection given clinically significant risks associated with use [see Dosage and Administration (2.2), Contraindications (4), Warnings and Precautions (5.1, 5.5, 5.7, 5.9)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
- Bupivacaine Hydrochloride Injection is not for intrathecal use.
- Discard unused portions of solution not containing preservatives, i.e., those supplied in single-dose vials, following initial use.
- Visually inspect this product for particulate matter and discoloration prior to administration whenever solution and container permit. Bupivacaine Hydrochloride Injection are clear, colorless solutions. Do not administer solutions which are discolored or contain particulate matter.
- Mixing or the prior or intercurrent use of any other local anesthetic with Bupivacaine Hydrochloride Injection is not recommended because of insufficient data on the clinical use of such mixtures.
Administration Precautions
- Bupivacaine Hydrochloride Injection Injection are to be administered in carefully adjusted dosages by or under the supervision of experienced clinicians who are well versed in the diagnosis and management of dose-related toxicity and other acute emergencies which might arise from the block to be employed.
- Use Bupivacaine Hydrochloride Injection only if the following are immediately available: oxygen, cardiopulmonary resuscitative equipment and drugs, and the personnel resources needed for proper management of toxic reactions and related emergencies [see Warnings and Precautions (5.2), Adverse Reactions (6), Overdosage (10)] .
- The toxic effects of local anesthetics are additive. Monitor for neurologic and cardiovascular effects related to local anesthetic systemic toxicity when additional local anesthetics are administered with Bupivacaine Hydrochloride Injection [see Warnings and Precautions (5.2), Drug Interactions (7.1), Overdosage (10)] .
- Aspirate for blood or cerebrospinal fluid (where applicable) prior to injecting Bupivacaine Hydrochloride Injection, both the initial dose and all subsequent doses, to avoid intravascular or intrathecal injection. However, a negative aspiration for blood or cerebrospinal fluid does not ensure against an intravascular or intrathecal injection [see Warnings and Precautions (5.9)] .
- Avoid rapid injection of a large volume of Bupivacaine Hydrochloride Injection and use fractional (incremental) doses when feasible.
- During major regional nerve blocks, such as those of the brachial plexus or lower extremity, the patient should have an indwelling intravenous catheter to assure adequate intravenous access. The lowest dosage of Bupivacaine Hydrochloride Injection that results in effective anesthesia should be used to avoid high plasma levels and serious adverse reactions.
- Perform careful and constant monitoring of cardiovascular and respiratory (adequacy of oxygenation and ventilation) vital signs and the patient's level of consciousness after each local anesthetic injection.
2.2 Recommended Concentrations and Dosages of Bupivacaine Hydrochloride Injection
The dosage of Bupivacaine Hydrochloride Injection administered varies with the anesthetic procedure, the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, the depth of anesthesia and degree of muscle relaxation required, the duration of anesthesia desired, individual tolerance, and the physical condition of the patient. Administer the smallest dosage and concentration required to produce the desired result.
The types of block and recommended Bupivacaine Hydrochloride Injection concentrations are shown in Table 1.
Table 1. Types of Block and Recommended Bupivacaine Hydrochloride Injection Concentrations
Type of Block
Bupivacaine Hydrochloride injection
0.25%
(2.5 mg/mL)0.5%
(5 mg/mL)0.75%
(7.5 mg/mL)*Local infiltration
✓
Peripheral nerve block
✓
✓
Retrobulbar block
✓
Sympathetic block
✓
Caudal block
✓
✓
Lumbar epidural block
✓
✓
✓
(not for obstetrical anesthesia)Epidural test dose
Dental block
* Bupivacaine Hydrochloride injection 0.75% (7.5 mg/mL) is not recommended for nonobstetrical surgical procedures in pregnant patients.
✓= indicated use [see Warnings and Precautions (5.1)].
At recommended dosages, Bupivacaine Hydrochloride produces complete sensory block, but the effect on motor function differs among the three concentrations. Table 2 provides information on the expected effect on motor function for the three concentrations.
Table 2. Types of Block and Recommended Bupivacaine Hydrochloride Injection Concentrations Bupivacaine Hydrochloride Injection Concentration
Motor Function
0.25%
(2.5 mg/mL)When used for caudal, epidural, or peripheral nerve block, produces incomplete motor block. Should be used for operations in which muscle relaxation is not important, or when another means of providing muscle relaxation is used concurrently. Onset of action may be slower than with the 0.5% (5 mg/mL) or 0.75% (7.5 mg/mL) solutions.
0.5%
(5 mg/mL)Provides motor blockade for caudal, epidural, or nerve block, but muscle relaxation may be inadequate for operations in which complete muscle relaxation is essential.
0.75%
(7.5 mg/mL)Produces complete motor block. Most useful for epidural block in abdominal operations requiring complete muscle relaxation, and for retrobulbar anesthesia. Not for obstetrical anesthesia.
The duration of anesthesia with Bupivacaine Hydrochloride Injection is such that for most indications, a single dose is sufficient.
The maximum dosage limit within the recommended dosage range must be individualized in each case after evaluating the size and physical status of the patient, as well as the anticipated rate of systemic absorption from a particular injection site.
The dosages in Table 3 are recommended as a guide for use in the average adult. These doses may be repeated once every three hours. Do not exceed a total daily dosage of 400 mg in 24 hours. The duration of anesthetic effect may be prolonged by the addition of epinephrine.
Table 3. Recommended Concentrations and Doses of Bupivacaine Hydrochloride Injection in Adults - *
- With continuous (intermittent) techniques, repeat doses increase the degree of motor block. The first repeat dose of 0.5% (5 mg/mL) may produce complete motor block. Intercostal nerve block with 0.25% (2.5 mg/mL) also may produce complete motor block for intra-thoracic and upper intra-abdominal surgery.
- †
- Solutions with or without epinephrine (i.e., applies to Bupivacaine Hydrochloride Injection.
- ‡
- For single-dose use; not for intermittent epidural technique. Not for obstetrical anesthesia.
Type of Block
Concentration of Bupivacaine Hydrochloride Injection
Each Dose
Motor Block*
mL
mg of Bupivacaine Hydrochloride Injection
Local infiltration
0.25%
(2.5 mg/mL) †Up to 70
(without epinephrine)Up to 175
(without epinephrine)―
Up to 90
(with epinephrine)Up to 225
(with epinephrine)Peripheral nerve block
0.5% (5 mg/mL) †
5–35
(without epinephrine)25–175
(without epinephrine)moderate to complete
5–45
(with epinephrine)25–225
(with epinephrine)0.25%
(2.5 mg/mL) †5–70
(without epinephrine)12.5–175
(without epinephrine)moderate to complete
5–90
(with epinephrine)12.5–225
(with epinephrine)Retrobulbar block
[see Dosage and Administration (2.6)]0.75%
(7.5 mg/mL)2–4
15–30
complete
Sympathetic block
0.25%
(2.5 mg/mL)20–50
50–125
―
Caudal block
[see Dosage and Administration (2.4)]0.5% (5 mg/mL) †
15–30
75–150
moderate to complete
0.25%
(2.5 mg/mL) †15–30
37.5–75
moderate
Lumbar epidural block
[see Dosage and Administration (2.3)]0.75%
(7.5 mg/mL) ‡10–20
75–150
complete
0.5% (5 mg/mL) †
10–20
50–100
moderate to complete
0.25%
(2.5 mg/mL) †10–20
25–50
partial to moderate
2.3 Use in Epidural Anesthesia
During epidural administration, administer Bupivacaine Hydrochloride Injection, 0.5% (5 mg/mL) and 0.75% (7.5 mg/mL) solutions in incremental doses of 3 mL to 5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal injection. Administer injections slowly, with frequent aspirations before and during the injection to avoid intravascular injection. Perform syringe aspirations before and during each supplemental injection in continuous (intermittent) catheter techniques. In obstetrics, use ONLY the 0.5% (5 mg/mL) and 0.25% (2.5 mg/mL) concentrations of Bupivacaine Hydrochloride Injection [see Warnings and Precautions (5.1)]; incremental doses of 3 mL to 5 mL of the 0.5% (5 mg/mL) solution not exceeding 50 mg to 100 mg at any dosing interval are recommended. Repeat doses should be preceded by a test dose containing epinephrine if not clinically contraindicated. Use only the single-dose vials for caudal or epidural anesthesia; avoid use of the multiple-dose vials for these procedures, which contain a preservative [see Dosage and Administration (2.1), Warnings and Precautions (5.9)] .
2.6 Use in Ophthalmic Surgery
When Bupivacaine Hydrochloride Injection 0.75% (7.5 mg/mL) is used for retrobulbar block, complete corneal anesthesia usually precedes onset of clinically acceptable external ocular muscle akinesia. Therefore, presence of akinesia rather than anesthesia alone should determine readiness of the patient for surgery [see Warnings and Precautions (5.15)] .
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Bupivacaine Hydrochloride Injection is contraindicated in:
- obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
- intravenous regional anesthesia (Bier Block) [see Warnings and Precautions (5.7)].
- patients with a known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride Injection.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Cardiac Arrest with Use of Bupivacaine Hydrochloride Injection in Obstetrical Anesthesia
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride Injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride Injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary.
5.2 Dose-Related Toxicity
The safety and effectiveness of Bupivacaine Hydrochloride Injection depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be performed after injection of Bupivacaine Hydrochloride Injection solutions.
Possible early warning signs of central nervous system (CNS) toxicity are restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, CNS depression, or drowsiness. Delay in proper management of dose-related toxicity, underventilation from any cause, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest, and, possibly, death.
During major regional nerve blocks, such as those of the brachial plexus or lower extremity, the patient should have an indwelling intravenous catheter to assure adequate intravenous access. Use the lowest dosage of Bupivacaine Hydrochloride Injection that results in effective anesthesia to avoid high plasma levels and serious adverse effects. Avoid rapid injection of a large volume of Bupivacaine Hydrochloride Injection solution and administer fractional (incremental) doses when feasible.
Injection of repeated doses of Bupivacaine Hydrochloride Injection may cause significant increases in plasma levels with each repeated dose due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with their age and physical status.
5.3 Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition [see Drug Interactions (7.5)] . If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious CNS and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Bupivacaine Hydrochloride Injection and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.5 Chondrolysis with Intra-Articular Infusion
Intra-articular infusions of local anesthetics including Bupivacaine Hydrochloride Injection following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are associated with chondrolysis. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2 ndmonth after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
5.7 Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block)
There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of Bupivacaine Hydrochloride Injection in this procedure is lacking. Therefore, Bupivacaine Hydrochloride Injection is contraindicated for use with this technique [see Contraindications (4)] .
5.9 Risk of Systemic Toxicities with Unintended Intravascular or Intrathecal Injection
Unintended intravascular or intrathecal injection of Bupivacaine Hydrochloride Injection may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Unintentional intrathecal injection during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column has resulted in underventilation or apnea ("Total or High Spinal"). A high spinal has been characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia [see Adverse Reactions (6)] .
Aspirate for blood or cerebrospinal fluid (where applicable) before injecting Bupivacaine Hydrochloride Injection, both the initial dose and all subsequent doses, to avoid intravascular or intrathecal injection. However, a negative aspiration for blood or cerebrospinal fluid does not ensure against an intravascular or intrathecal injection.
5.10 Risk of Toxicity in Patients with Hepatic Impairment
Because amide local anesthetics such as bupivacaine are metabolized by the liver, consider reduced dosing and increased monitoring for bupivacaine systemic toxicity in patients with moderate to severe hepatic impairment who are treated with Bupivacaine Hydrochloride Injection, especially with repeat doses [see Use in Specific Populations (8.6)].
5.11 Risk of Use in Patients with Impaired Cardiovascular Function
Bupivacaine Hydrochloride Injection should be given in reduced doses in patients with impaired cardiovascular function (e.g., hypotension, heartblock) because they may be less able to compensate for functional changes associated with the prolongation of AV conduction produced by Bupivacaine Hydrochloride Injection. Monitor patients closely for blood pressure, heart rate, and ECG changes.
5.14 Risk of Adverse Reactions with Use in Head and Neck Area
Small doses of local anesthetics (e.g., Bupivacaine Hydrochloride Injection) injected into the head and neck area, including retrobulbar, dental, and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. The injection procedures require the utmost care. Confusion, convulsions, respiratory depression, and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. They may also be due to puncture of the dural sheath of the optic nerve during retrobulbar block with diffusion of any local anesthetic along the subdural space to the midbrain. Monitor circulation and respiration and constantly observe patients receiving Bupivacaine Hydrochloride Injection blocks. Resuscitative equipment and drugs, and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded [see Dosage and Administration (2.2)].
5.15 Risk of Respiratory Arrest with Use in Ophthalmic Surgery
Clinicians who perform retrobulbar blocks should be aware that there have been reports of respiratory arrest following local anesthetic injection. Prior to retrobulbar block (e.g., with Bupivacaine Hydrochloride Injection), as with all other regional procedures, resuscitative equipment and drugs, and personnel to manage respiratory arrest or depression, convulsions, and cardiac stimulation or depression should be immediately available [see Warnings and Precautions (5.14)]. As with other anesthetic procedures, patients should be constantly monitored following ophthalmic blocks for signs of these adverse reactions, which may occur following relatively low total doses.
A concentration of 0.75% bupivacaine is indicated for retrobulbar block; however, this concentration is not indicated for any other peripheral nerve block, including the facial nerve, and not indicated for local infiltration, including the conjunctiva [see Indications and Usage (1)].
5.16 Risk of Inadvertent Trauma to Tongue, Lips, and Buccal Mucosa in Dental Applications
Because of the long duration of anesthesia, when Bupivacaine Hydrochloride injection with epinephrine [0.5% (5 mg/mL) of bupivacaine] is used for dental injections, warn patients about the possibility of inadvertent trauma to tongue, lips, and buccal mucosa and advise them not to chew solid foods until sensation returns [see Patient Counseling Information (17)] .
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions have been reported and described in the Warnings and Precautions section of the labeling:
- Cardiac Arrest in Obstetrical Anesthesia [see Warnings and Precautions (5.1)]
- Dose-Related Toxicity [see Warnings and Precautions (5.2)]
- Methemoglobinemia [see Warnings and Precautions (5.3)]
- Chondrolysis with Intra-Articular Infusion [see Warnings and Precautions (5.5)]
- Cardiac Arrest with Intravenous Regional Anesthesia Use [see Contraindications (4), Warnings and Precautions (5.7)]
- Systemic Toxicities with Unintended Intravascular or Intrathecal Injection [see Warnings and Precautions (5.9)]
- Respiratory Arrest Following Retrobulbar Block [see Warnings and Precautions (5.15)]
The following adverse reactions from voluntary reports or clinical studies have been reported with bupivacaine. Because many of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions to Bupivacaine Hydrochloride Injection are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation.
The most commonly encountered acute adverse reactions that demand immediate counter-measures were related to the CNS and the cardiovascular system. These adverse reactions were generally dose-related and due to high plasma levels which may have resulted from overdosage, rapid absorption from the injection site, diminished tolerance, or from unintentional intravascular injection of the local anesthetic solution. In addition to systemic dose-related toxicity, unintentional intrathecal injection of drug during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column (especially in the head and neck region) has resulted in underventilation or apnea ("Total or High Spinal"). Also, hypotension due to loss of sympathetic tone and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia have occurred. This has led to secondary cardiac arrest when untreated.
Nervous System Disorders
Adverse reactions were characterized by excitation and/or depression of the central nervous system and included restlessness, anxiety, dizziness, tinnitus, blurred vision, tremors, convulsions, drowsiness, unconsciousness, respiratory arrest, nausea, vomiting, chills, pupillary constriction.
In the practice of caudal or lumbar epidural block, unintentional penetration of the subarachnoid space by the catheter or needle has occurred. Subsequent adverse effects may have depended partially on the amount of drug administered intrathecally and the physiological and physical effects of a dural puncture. A high spinal has been characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia.
Neurologic effects following epidural or caudal anesthesia have included spinal block of varying magnitude (including high or total spinal block); hypotension secondary to spinal block; urinary retention; fecal and urinary incontinence; loss of perineal sensation and sexual function; persistent anesthesia, paresthesia, weakness, paralysis of the lower extremities and loss of sphincter control, all of which had slow, incomplete, or no recovery; headache; backache; septic meningitis; meningismus; slowing of labor; increased incidence of forceps delivery; and cranial nerve palsies due to traction on nerves from loss of cerebrospinal fluid.
Neurologic effects following other procedures or routes of administration have included persistent anesthesia, paresthesia, weakness, paralysis, all with slow, incomplete, or no recovery.
Convulsions: Incidence varied with the procedure used and the total dose administered. In a survey of studies of epidural anesthesia, overt toxicity progressing to convulsions occurred in approximately 0.1% of local anesthetic administrations. The incidences of adverse neurologic reactions associated with the use of local anesthetics may be related to the total dose of local anesthetic administered and are also dependent upon the particular drug used, the route of administration, and the physical status of the patient.
Cardiac Disorders
High doses or unintentional intravascular injection have led to high plasma levels and related depression of the myocardium, decreased cardiac output, heartblock, hypotension, bradycardia, ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation, and cardiac arrest [see Warnings and Precautions (5.9)].
Immune System Disorders
Allergic-type reactions have occurred as a result of sensitivity to bupivacaine or to other formulation ingredients, such as the antimicrobial preservative methylparaben contained in multiple-dose vials or sulfites in epinephrine-containing solutions. These reactions were characterized by signs such as urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), tachycardia, sneezing, nausea, vomiting, dizziness, syncope, excessive sweating, elevated temperature, and severe hypotension. Cross sensitivity among members of the amide-type local anesthetic group has been reported [see Warnings and Precautions (5.8)] .
-
7 DRUG INTERACTIONS
7.1 Local Anesthetics
The toxic effects of local anesthetics are additive. If coadministration of other local anesthetics with Bupivacaine Hydrochloride Injection cannot be avoided, monitor patients for neurologic and cardiovascular effects related to local anesthetic systemic toxicity [see Dosage and Administration (2.1), Warnings and Precautions (5.2)] .
7.5 Drugs Associated with Methemoglobinemia
Patients who are administered Bupivacaine Hydrochloride Injection are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics [see Warnings and Precautions (5.3)] .
Examples of Drugs Associated with Methemoglobinemia: Class Examples Nitrates/Nitrites
nitric oxide, nitroglycerin, nitroprusside, nitrous oxide
Local anesthetics
articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine
Antineoplastic agents
cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase
Antibiotics
dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides
Antimalarials
chloroquine, primaquine
Anticonvulsants
phenobarbital, phenytoin, sodium valproate
Other drugs
acetaminophen, metoclopramide, quinine, sulfasalazine
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Bupivacaine Hydrochloride Injection is contraindicated for obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death [see Contraindications (4), Warnings and Precautions (5.1)].
There are no available data on use of Bupivacaine Hydrochloride Injection in pregnant women to inform a drug-associated risk of adverse developmental outcomes.
In animal studies, embryo-fetal lethality was noted when bupivacaine was administered subcutaneously to pregnant rabbits during organogenesis at clinically relevant doses. Decreased pup survival was observed in a rat pre- and post-natal developmental study (dosing from implantation through weaning) at a dose level comparable to the daily maximum recommended human dose (MRHD) on a body surface area (BSA) basis. Based on animal data, advise pregnant women of the potential risks to a fetus (see Data).
Local anesthetics rapidly cross the placenta, and when used for epidural, caudal, or pudendal block anesthesia, can cause varying degrees of maternal, fetal, and neonatal toxicity [see Clinical Pharmacology (12.3)]. The incidence and degree of toxicity depend upon the procedure performed, the type, and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus, and neonate involve alterations of the CNS, peripheral vascular tone, and cardiac function.
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, inform the patient of the potential hazard to the fetus. The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Maternal Adverse Reactions
Maternal hypotension has resulted from regional anesthesia. Local anesthetics produce vasodilation by blocking sympathetic nerves. The supine position is dangerous in pregnant women at term because of aortocaval compression by the gravid uterus. Therefore, during treatment of systemic toxicity, maternal hypotension or fetal bradycardia following regional block, the parturient should be maintained in the left lateral decubitus position if possible, or manual displacement of the uterus off the great vessels be accomplished. Elevating the patient's legs will also help prevent decreases in blood pressure. The fetal heart rate also should be monitored continuously and electronic fetal monitoring is highly advisable.
Labor or Delivery
Epidural, caudal, or pudendal anesthesia may alter the forces of parturition through changes in uterine contractility or maternal expulsive efforts. Epidural anesthesia has been reported to prolong the second stage of labor by removing the parturient's reflex urge to bear down or by interfering with motor function. The use of obstetrical anesthesia may increase the need for forceps assistance.
The use of some local anesthetic drug products during labor and delivery may be followed by diminished muscle strength and tone for the first day or two of life. This has not been reported with bupivacaine.
It is extremely important to avoid aortocaval compression by the gravid uterus during administration of regional block to parturients. To do this, the patient must be maintained in the left lateral decubitus position or a blanket roll or sandbag may be placed beneath the right hip and gravid uterus displaced to the left.
Animal Data
Bupivacaine hydrochloride produced developmental toxicity when administered subcutaneously to pregnant rats and rabbits at clinically relevant doses.
Bupivacaine hydrochloride was administered subcutaneously to rats at doses of 4.4, 13.3, & 40 mg/kg and to rabbits at doses of 1.3, 5.8, & 22.2 mg/kg during the period of organogenesis (implantation to closure of the hard palate). The high doses are comparable to the daily MRHD of 400 mg/day on a mg/m 2BSA basis. No embryo-fetal effects were observed in rats at the high dose which caused increased maternal lethality. An increase in embryo-fetal deaths was observed in rabbits at the high dose in the absence of maternal toxicity with the fetal No Observed Adverse Effect Level representing approximately 0.3 times the MRHD on a BSA basis.
In a rat pre- and post-natal developmental study (dosing from implantation through weaning) conducted at subcutaneous doses of 4.4, 13.3, & 40 mg/kg, decreased pup survival was observed at the high dose. The high dose is comparable to the daily MRHD of 400 mg/day on a BSA basis.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted with bupivacaine. Bupivacaine has been reported to be excreted in human milk suggesting that the nursing infant could be theoretically exposed to a dose of the drug. Bupivacaine Hydrochloride Injection should be administered to lactating women only if clearly indicated. Studies assessing the effects of Bupivacaine Hydrochloride Injection in breastfed children have not been performed. Studies to assess the effect of Bupivacaine Hydrochloride Injection on milk production or excretion have not been performed. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for bupivacaine and any potential adverse effects on the breastfed child from bupivacaine or from the underlying maternal condition.
8.4 Pediatric Use
Bupivacaine Hydrochloride Injection is approved for use in adults. Administration of Bupivacaine Hydrochloride Injection in pediatric patients younger than 12 years is not recommended.
Continuous infusions of bupivacaine in pediatric patients have been reported to result in high systemic levels of bupivacaine and seizures; high plasma levels may also be associated with cardiovascular abnormalities.
8.5 Geriatric Use
Patients 65 years and over, particularly those with hypertension, may be at increased risk for developing hypotension while undergoing anesthesia with Bupivacaine Hydrochloride Injection.
In clinical studies of bupivacaine, elderly patients reached the maximal spread of analgesia and maximal motor blockade more rapidly than younger adult patients.
Differences in various pharmacokinetic parameters have been observed between elderly and younger adult patients [see Clinical Pharmacology (12.3)].
This product is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Elderly patients may require lower doses of Bupivacaine Hydrochloride Injection.
8.6 Hepatic Impairment
Amide-type local anesthetics, such as bupivacaine, are metabolized by the liver. Patients with severe hepatic impairment, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations, and potentially local anesthetic systemic toxicity. Therefore, consider reduced dosing and increased monitoring for local anesthetic systemic toxicity in patients with moderate to severe hepatic impairment treated with Bupivacaine Hydrochloride Injection, especially with repeat doses [see Warnings and Precautions (5.10)] .
8.7 Renal Impairment
Bupivacaine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with renal impairment. This should be considered when selecting the Bupivacaine Hydrochloride Injection dosage [see Use in Specific Populations (8.5)] .
-
10 OVERDOSAGE
Clinical Presentation
Acute emergencies from use of Bupivacaine Hydrochloride Injection are generally related to high plasma levels encountered during therapeutic use or to unintended intrathecal injection [see Warnings and Precautions (5.2, 5.9), Adverse Reactions (6)].
If not treated immediately, convulsions with simultaneous hypoxia, hypercarbia, and acidosis plus myocardial depression from the direct effects of bupivacaine may result in cardiac arrhythmias, bradycardia, asystole, ventricular fibrillation, or cardiac arrest. Respiratory abnormalities, including apnea, may occur. Hypoventilation or apnea due to unintentional intrathecal injection of Bupivacaine Hydrochloride Injection may produce these same signs and also lead to cardiac arrest if ventilatory support is not instituted. If cardiac arrest should occur, successful outcome may require prolonged resuscitative efforts.
Management
The first step in the management of systemic toxic reactions, as well as hypoventilation or apnea due to unintentional intrathecal injection of Bupivacaine Hydrochloride Injection, consists of immediateattention to the establishment and maintenance of a patent airway and effective assisted or controlled ventilation with 100% oxygen with a delivery system capable of permitting immediate positive airway pressure by mask. Endotracheal intubation, using drugs and techniques familiar to the clinician, may be indicated after initial administration of oxygen by mask if difficulty is encountered in the maintenance of a patent airway, or if prolonged ventilatory support (assisted or controlled) is indicated.
If necessary, use drugs to manage the convulsions. A bolus intravenous dose of a benzodiazepine will counteract CNS stimulation related to Bupivacaine Hydrochloride Injection. Immediately after the institution of ventilatory measures, evaluate the adequacy of the circulation. Supportive treatment of circulatory depression may require Advanced Cardiac Life Support measures.
-
11 DESCRIPTION
Bupivacaine Hydrochloride Injection contains bupivacaine hydrochloride, an amide local anesthetic, as the active pharmaceutical ingredient. The route of administration for Bupivacaine Hydrochloride Injection is by injection, for infiltration, perineural, caudal, epidural, or retrobulbar use. [see Warnings and Precautions (5.4)] .
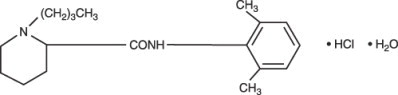
Bupivacaine hydrochloride is 2-piperidinecarboxamide, 1-butyl- N-(2,6-dimethylphenyl)-, monohydrochloride, monohydrate. It is a white crystalline powder that is freely soluble in 95 percent ethanol, soluble in water, and slightly soluble in chloroform or acetone. It has the following structural formula:
Bupivacaine Hydrochloride Injection, USP is a clear and colorless sterile isotonic solution. Each mL of single-dose vial contains 5 mg of bupivacaine hydrochloride (equivalent to 4.44 mg of bupivacaine, respectively), sodium chloride for isotonicity, sodium hydroxide or hydrochloric acid to adjust the pH between 4 and 6.5, in water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bupivacaine blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination, and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
12.2 Pharmacodynamics
Systemic absorption of bupivacaine produces effects on the cardiovascular system and CNS. At blood concentrations achieved with normal therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. These cardiovascular changes are more likely to occur after unintended intravascular injection of bupivacaine [see Warnings and Precautions (5.9)] .
Following systemic absorption, bupivacaine can produce CNS stimulation, CNS depression, or both. Apparent central stimulation is manifested as restlessness, tremors, and shivering, progressing to convulsions, followed by CNS depression and coma progressing ultimately to respiratory arrest. However, bupivacaine has a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited state.
The duration of local anesthesia after administration of Bupivacaine Hydrochloride Injection is longer than that observed after administration of other commonly used short-acting local anesthetics. There appears to be a period of analgesia that persists after the resolution of the block and return of sensation.
The onset of action following dental injections is usually 2 to 10 minutes and may last up to 7 hours.
12.3 Pharmacokinetics
Systemic plasma levels of bupivacaine following administration of Bupivacaine Hydrochloride Injection do not correlate with local efficacy.
Absorption
The rate of systemic absorption of bupivacaine is dependent upon the total dose and concentration of drug administered, the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic solution. A dilute concentration of epinephrine (1:200,000) usually reduces the rate of absorption and peak plasma concentration of bupivacaine, permitting the use of moderately larger total doses and sometimes prolonging the duration of action [see Dosage and Administration (2)] .
After injection of Bupivacaine Hydrochloride Injection for caudal, epidural, or peripheral nerve block, peak levels of bupivacaine in the blood are reached in 30 to 45 minutes, followed by a decline to insignificant levels during the next three to six hours.
Distribution
Bupivacaine appears to cross the placenta by passive diffusion. The rate and degree of diffusion is governed by (1) the degree of plasma protein binding, (2) the degree of ionization, and (3) the degree of lipid solubility. Fetal/maternal ratios of bupivacaine appear to be inversely related to the degree of plasma protein binding, because only the free, unbound drug is available for placental transfer. Bupivacaine with a high protein binding capacity (95%) has a low fetal/maternal ratio (0.2 to 0.4). The extent of placental transfer is also determined by the degree of ionization and lipid solubility of the drug. Lipid soluble, nonionized drugs readily enter the fetal blood from the maternal circulation.
Depending upon the route of administration, bupivacaine is distributed to some extent to all body tissues, with high concentrations found in highly perfused organs such as the liver, lungs, heart, and brain.
Pharmacokinetic studies on the plasma profile of bupivacaine after direct intravenous injection (Bupivacaine Hydrochloride Injection is not approved for intravenous use) suggest a three-compartment open model. The first compartment is represented by the rapid intravascular distribution of the drug. The second compartment represents the equilibration of the drug throughout the highly perfused organs such as the brain, myocardium, lungs, kidneys, and liver. The third compartment represents an equilibration of the drug with poorly perfused tissues, such as muscle and fat.
Elimination
The half-life of bupivacaine in adults is 2.7 hours.
Metabolism
Amide-type local anesthetics such as bupivacaine are metabolized primarily in the liver via conjugation with glucuronic acid. Pipecoloxylidine is the major metabolite of bupivacaine. The elimination of drug from tissue distribution depends largely upon the availability of binding sites in the circulation to carry it to the liver where it is metabolized.
Specific Populations
Geriatric Patients
Elderly patients exhibited higher peak plasma concentrations than younger patients following administration of Bupivacaine Hydrochloride Injection. The total plasma clearance was decreased in these patients [see Use in Specific Populations (8.5)] .
Patients with Hepatic Impairment
Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of hepatic disease. Patients with hepatic disease, especially those with severe hepatic disease, may be more susceptible to the potential toxicities of the amide-type local anesthetics [see Use in Specific Populations (8.6)] .
Patients with Renal Impairment
Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of renal disease, factors affecting urinary pH, and renal blood flow [see Use in Specific Populations (8.5, 8.7)] .
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Store at 20 °C to 25 °C (68 °F to 77 °F); excursions permitted between 15 °C to 30 °C (59 °F to 86 °F). [See USP Controlled Room Temperature.]
Bupivacaine Hydrochloride Injection, USP─ Solutions of bupivacaine hydrochloride may be autoclaved. Autoclave at 15-pound pressure, 121 °C (250 °F) for 15 minutes. This product is clear and colorless. Do not use the solution if it is discolored or if it contains a precipitate.
Unit of Sale
Concentration
NDC 0409-1162-01
Tray of 25 single-dose teartop vials0.5%
50 mg/10 mL
(5 mg/mL)NDC 0409-1162-02
Tray of 25 single-dose teartop vials0.5%
150 mg/30 mL
(5 mg/mL)For single-dose vials: Discard unused portion.
-
17 PATIENT COUNSELING INFORMATION
Allergic-Type Reactions
Assess if the patient has had allergic-type reactions to amide-type local anesthetics [see Contraindications (4), Adverse Reactions (6)].
Temporary Loss of Sensation and Motor Activity After Caudal or Epidural Anesthesia
When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity, usually in the lower half of the body, following proper administration of caudal or epidural anesthesia.
Instructions After Dental Injection of Bupivacaine Hydrochloride Injection
Advise patients receiving dental injections of Bupivacaine Hydrochloride Injection not to chew solid foods or to test the anesthetized area by biting or probing until anesthesia has worn off (up to 7 hours) [see Warnings and Precautions (5.16)].
Methemoglobinemia
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue [see Warnings and Precautions (5.3)].
-
SPL UNCLASSIFIED SECTION
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1176-6.0
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Lidocaine hydrochloride injection, USP is sterile, nonpyrogenic, aqueous solution that contains a local anesthetic agent and is administered parenterally by injection. See INDICATIONS AND USAGEsection for specific uses.
Lidocaine hydrochloride injection, USP contains lidocaine hydrochloride, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride and has the molecular weight 270.8. Lidocaine hydrochloride (C 14H 22N 2O • HCl) has the following structural formula:
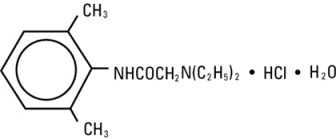
Lidocaine hydrochloride injection, USP is a sterile, nonpyrogenic, isotonic solution containing sodium chloride. The pH of the solution is adjusted to approximately 6.5 (5.0 to 7.0) with sodium hydroxide and/or hydrochloric acid.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Lidocaine hydrochloride stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses thereby effecting local anesthetic action.
Hemodynamics
Excessive blood levels may cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. With central neural blockade these changes may be attributable to block of autonomic fibers, a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system, and/or the beta-adrenergic receptor stimulating action of epinephrine when present. The net effect is normally a modest hypotension when the recommended dosages are not exceeded.
Pharmacokinetics and Metabolism
Information derived from diverse formulations, concentrations and usages reveals that lidocaine hydrochloride is completely absorbed following parenteral administration, its rate of absorption depending, for example, upon various factors such as the site of administration and the presence or absence of a vasoconstrictor agent. Except for intravascular administration, the highest blood levels are obtained following intercostal nerve block and the lowest after subcutaneous administration.
The plasma binding of lidocaine hydrochloride is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg of free base per mL 60 to 80 percent of lidocaine hydrochloride is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein.
Lidocaine hydrochloride crosses the blood-brain and placental barriers, presumably by passive diffusion.
Lidocaine hydrochloride is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine hydrochloride. Approximately 90% of lidocaine hydrochloride administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6-dimethylaniline.
The elimination half-life of lidocaine hydrochloride following an intravenous bolus injection is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine hydrochloride is metabolized, any condition that affects liver function may alter lidocaine hydrochloride kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine hydrochloride kinetics but may increase the accumulation of metabolites.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine hydrochloride required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 mcg free base per mL. In the rhesus monkey arterial blood levels of 18 to 21 mcg/mL have been shown to be threshold for convulsive activity. -
INDICATIONS AND USAGE
Lidocaine hydrochloride injection is indicated for production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous regional anesthesia by peripheral nerve block techniques such as brachial plexus and intercostal and by central neural techniques such as lumbar and caudal epidural blocks, when the accepted procedures for these techniques as described in standard textbooks are observed.
- CONTRAINDICATIONS
-
WARNINGS
LIDOCAINE HYDROCHLORIDE INJECTION FOR INFILTRATION AND NERVE BLOCK SHOULD BE EMPLOYED ONLY BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE EMERGENCIES THAT MIGHT ARISE FROM THE BLOCK TO BE EMPLOYED AND THEN ONLY AFTER ENSURING THE IMMEDIATEAVAILABILITY OF OXYGEN, OTHER RESUSCITATIVE DRUGS, CARDIOPULMONARY EQUIPMENT AND THE PERSONNEL NEEDED FOR PROPER MANAGEMENT OF TOXIC REACTIONS AND RELATED EMERGENCIES (see also ADVERSE REACTIONSand PRECAUTIONS). DELAY IN PROPER MANAGEMENT OF DOSE-RELATED TOXICITY, UNDERVENTILATION FROM ANY CAUSE AND/OR ALTERED SENSITIVITY MAY LEAD TO THE DEVELOPMENT OF ACIDOSIS, CARDIAC ARREST AND, POSSIBLY, DEATH.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue lidocaine hydrochloride and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2 ndmonth after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
To avoid intravascular injection, aspiration should be performed before the local anesthetic solution is injected. The needle must be repositioned until no return of blood can be elicited by aspiration. Note, however, that the absence of blood in the syringe does not guarantee that intravascular injection has been avoided.
Anaphylactic reactions may occur following administration of lidocaine hydrochloride (see ADVERSE REACTIONS).
In the case of severe reaction, discontinue the use of the drug. -
PRECAUTIONS
General
The safety and effectiveness of lidocaine hydrochloride depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be consulted for specific techniques and precautions for various regional anesthetic procedures.
Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use (see WARNINGSand ADVERSE REACTIONS). The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Syringe aspirations should also be performed before and during each supplemental injection when using indwelling catheter techniques. During the administration of epidural anesthesia, it is recommended that a test dose be administered initially and that the patient be monitored for central nervous system toxicity and cardiovascular toxicity, as well as for signs of unintended intrathecal administration, before proceeding. When clinical conditions permit, consideration should be given to employing local anesthetic solutions that contain epinephrine for the test dose because circulatory changes compatible with epinephrine may also serve as a warning sign of unintended intravascular injection. An intravascular injection is still possible even if aspirations for blood are negative. Repeated doses of lidocaine hydrochloride may cause significant increases in blood levels with each repeated dose because of slow accumulation of the drug or its metabolites. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age and physical condition. Lidocaine hydrochloride should also be used with caution in patients with severe shock or heart block.
Lumbar and caudal epidural anesthesia should be used with extreme caution in persons with the following conditions: existing neurological disease, spinal deformities, septicemia, and severe hypertension.
Local anesthetic solutions containing a vasoconstrictor should be used cautiously and in carefully circumscribed quantities in areas of the body supplied by end arteries or having otherwise compromised blood supply. Patients with peripheral vascular disease and those with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response. Ischemic injury or necrosis may result. Preparations containing a vasoconstrictor should be used with caution in patients during or following the administration of potent general anesthetic agents, since cardiac arrhythmias may occur under such conditions.
Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of consciousness should be accomplished after each local anesthetic injection. It should be kept in mind at such times that restlessness, anxiety, tinnitus, dizziness, blurred vision, tremors, depression or drowsiness may be early warning signs of central nervous system toxicity.
Since amide-type local anesthetics are metabolized by the liver, lidocaine hydrochloride injection should be used with caution in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at greater risk of developing toxic plasma concentrations. Lidocaine hydrochloride injection should also be used with caution in patients with impaired cardiovascular function since they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia. Since it is not known whether amide-type local anesthetics may trigger this reaction and since the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for the management of malignant hyperthermia should be available. Early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and institution of treatment, including oxygen therapy, indicated supportive measures and dantrolene (consult dantrolene sodium intravenous package insert before using).
Proper tourniquet technique, as described in publications and standard textbooks, is essential in the performance of intravenous regional anesthesia. Solutions containing epinephrine or other vasoconstrictors should not be used for this technique.
Lidocaine hydrochloride should be used with caution in persons with known drug sensitivities. Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross-sensitivity to lidocaine hydrochloride.Use in the Head and Neck Area
Small doses of local anesthetics injected into the head and neck area, including retrobulbar, dental and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Confusion, convulsions, respiratory depression and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded (see DOSAGE AND ADMINISTRATION).
Information for Patients
When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity, usually in the lower half of the body, following proper administration of epidural anesthesia.
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.Clinically Significant Drug Interactions
The administration of local anesthetic solutions containing epinephrine or norepinephrine to patients receiving monoamine oxidase inhibitors or tricyclic antidepressants may produce severe, prolonged hypertension.
Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine.
Concurrent use of these agents should generally be avoided. In situations when concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs (for the treatment of hypotension related to obstetric blocks) and ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.Drug/Laboratory Test Interactions
The intramuscular injection of lidocaine hydrochloride may result in an increase in creatine phosphokinase levels. Thus, the use of this enzyme determination, without isoenzyme separation, as a diagnostic test for the presence of acute myocardial infarction may be compromised by the intramuscular injection of lidocaine hydrochloride.
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia: Class
Examples
Nitrates/Nitrites
nitric oxide, nitroglycerin, nitroprusside, nitrous oxide
Local anesthetics
articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine
Antineoplastic agents
cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase
Antibiotics
dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides
Antimalarials
chloroquine, primaquine
Anticonvulsants
Phenobarbital, phenytoin, sodium valproate
Other drugs
acetaminophen, metoclopramide, quinine, sulfasalazine
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of lidocaine hydrochloride in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Pregnancy
Teratogenic Effects
Reproduction studies have been performed in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine hydrochloride. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine hydrochloride to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
Labor and Delivery
Local anesthetics rapidly cross the placenta and when used for epidural, paracervical, pudendal or caudal block anesthesia, can cause varying degrees of maternal, fetal and neonatal toxicity (see CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism). The potential for toxicity depends upon the procedure performed, the type and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus and neonate involve alterations of the central nervous system, peripheral vascular tone and cardiac function.
Maternal hypotension has resulted from regional anesthesia. Local anesthetics produce vasodilation by blocking sympathetic nerves. Elevating the patient’s legs and positioning her on her left side will help prevent decreases in blood pressure.
The fetal heart rate also should be monitored continuously, and electronic fetal monitoring is highly advisable.
Epidural, spinal, paracervical, or pudendal anesthesia may alter the forces of parturition through changes in uterine contractility or maternal expulsive efforts. In one study, paracervical block anesthesia was associated with a decrease in the mean duration of first stage labor and facilitation of cervical dilation. However, spinal and epidural anesthesia have also been reported to prolong the second stage of labor by removing the parturient’s reflex urge to bear down or by interfering with motor function. The use of obstetrical anesthesia may increase the need for forceps assistance.
The use of some local anesthetic drug products during labor and delivery may be followed by diminished muscle strength and tone for the first day or two of life. The long-term significance of these observations is unknown. Fetal bradycardia may occur in 20 to 30 percent of patients receiving paracervical nerve block anesthesia with the amide-type local anesthetics and may be associated with fetal acidosis. Fetal heart rate should always be monitored during paracervical anesthesia. The physician should weigh the possible advantages against risks when considering a paracervical block in prematurity, toxemia of pregnancy, and fetal distress. Careful adherence to recommended dosage is of the utmost importance in obstetrical paracervical block. Failure to achieve adequate analgesia with recommended doses should arouse suspicion of intravascular or fetal intracranial injection. Cases compatible with unintended fetal intracranial injection of local anesthetic solution have been reported following intended paracervical or pudendal block or both. Babies so affected present with unexplained neonatal depression at birth, which correlates with high local anesthetic serum levels, and often manifest seizures within six hours. Prompt use of supportive measures combined with forced urinary excretion of the local anesthetic has been used successfully to manage this complication.
Case reports of maternal convulsions and cardiovascular collapse following use of some local anesthetics for paracervical block in early pregnancy (as anesthesia for elective abortion) suggest that systemic absorption under these circumstances may be rapid. The recommended maximum dose of each drug should not be exceeded. Injection should be made slowly and with frequent aspiration. Allow a 5-minute interval between sides. -
ADVERSE REACTIONS
Systemic
Adverse experiences following the administration of lidocaine hydrochloride are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage, rapid absorption or inadvertent intravascular injection, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central Nervous System
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest.
Drowsiness following the administration of lidocaine hydrochloride is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular System
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity to local anesthetic agents. Allergic reactions, including anaphylactic reactions, may occur as a result of sensitivity to lidocaine, but are infrequent. If allergic reactions do occur, they should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
There have been no reports of cross sensitivity between lidocaine hydrochloride and procainamide or between lidocaine hydrochloride and quinidine.Neurologic
The incidences of adverse reactions associated with the use of local anesthetics may be related to the total dose of local anesthetic administered and are also dependent upon the particular drug used, the route of administration and the physical status of the patient. In a prospective review of 10,440 patients who received lidocaine hydrochloride for spinal anesthesia, the incidences of adverse reactions were reported to be about 3 percent each for positional headaches, hypotension and backache; 2 percent for shivering; and less than 1 percent each for peripheral nerve symptoms, nausea, respiratory inadequacy and double vision. Many of these observations may be related to local anesthetic techniques, with or without a contribution from the local anesthetic.
In the practice of caudal or lumbar epidural block, occasional unintentional penetration of the subarachnoid space by the catheter may occur. Subsequent adverse effects may depend partially on the amount of drug administered subdurally. These may include spinal block of varying magnitude (including total spinal block), hypotension secondary to spinal block, loss of bladder and bowel control, and loss of perineal sensation and sexual function. Persistent motor, sensory and/or autonomic (sphincter control) deficit of some lower spinal segments with slow recovery (several months) or incomplete recovery have been reported in rare instances when caudal or lumbar epidural block has been attempted. Backache and headache have also been noted following use of these anesthetic procedures.
There have been reported cases of permanent injury to extraocular muscles requiring surgical repair following retrobulbar administration. -
OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local anesthetic solution (see ADVERSE REACTIONS, WARNINGS, and PRECAUTIONS).
Management of Local Anesthetic Emergencies
The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient’s state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
The first step in the management of convulsions, as well as underventilation or apnea due to unintended subarachnoid injection of drug solution, consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to the use of local anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor as directed by the clinical situation (e.g., ephedrine).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. Underventilation or apnea due to unintentional subarachnoid injection of local anesthetic solution may produce these same signs and also lead to cardiac arrest if ventilatory support is not instituted. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated, after initial administration of oxygen by mask, if difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or controlled) is indicated.
Dialysis is of negligible value in the treatment of acute overdosage with lidocaine hydrochloride.
The oral LD 50of lidocaine hydrochloride in non-fasted female rats is 459 (346 to 773) mg/kg (as the salt) and 214 (159 to 324) mg/kg (as the salt) in fasted female rats. -
DOSAGE AND ADMINISTRATION
Table 1 (Recommended Dosages) summarizes the recommended volumes and concentrations of lidocaine hydrochloride injection for various types of anesthetic procedures. The dosages suggested in this table are for normal healthy adults and refer to the use of epinephrine-free solutions. When larger volumes are required, only solutions containing epinephrine should be used except in those cases where vasopressor drugs may be contraindicated.
There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures. Lidocaine hydrochloride injection is not approved for this use (see WARNINGSand DOSAGE AND ADMINISTRATION).
These recommended doses serve only as a guide to the amount of anesthetic required for most routine procedures. The actual volumes and concentrations to be used depend on a number of factors such as type and extent of surgical procedure, depth of anesthesia and degree of muscular relaxation required, duration of anesthesia required, and the physical condition of the patient. In all cases the lowest concentration and smallest dose that will produce the desired result should be given. Dosages should be reduced for children and for the elderly and debilitated patients and patients with cardiac and/or liver disease.
The onset of anesthesia, the duration of anesthesia and the degree of muscular relaxation are proportional to the volume and concentration (i.e., total dose) of local anesthetic used. Thus, an increase in volume and concentration of lidocaine hydrochloride injection will decrease the onset of anesthesia, prolong the duration of anesthesia, provide a greater degree of muscular relaxation and increase the segmental spread of anesthesia. However, increasing the volume and concentration of lidocaine hydrochloride injection may result in a more profound fall in blood pressure when used in epidural anesthesia. Although the incidence of side effects with lidocaine hydrochloride is quite low, caution should be exercised when employing large volumes and concentrations, since the incidence of side effects is directly proportional to the total dose of local anesthetic agent injected.Epidural Anesthesia
For epidural anesthesia the following dosage form of lidocaine hydrochloride injection is recommended:
1% without epinephrine 30 mL single dose vials
Although this solution is intended specifically for epidural anesthesia, it may also be used for infiltration and peripheral nerve block, provided it is employed as a single dose unit.
This solution contains no bacteriostatic agent.
In epidural anesthesia, the dosage varies with the number of dermatomes to be anesthetized (generally 2 to 3 mL of the indicated concentration per dermatome).Caudal and Lumbar Epidural Block
As a precaution against the adverse experience sometimes observed following unintentional penetration of the subarachnoid space, a test dose such as 2 to 3 mL of 1.5% lidocaine hydrochloride should be administered at least 5 minutes prior to injecting the total volume required for a lumbar or caudal epidural block. The test dose should be repeated if the patient is moved in a manner that may have displaced the catheter. Epinephrine, if contained in the test dose (10 to 15 mcg have been suggested), may serve as a warning of unintentional intravascular injection. If injected into a blood vessel, this amount of epinephrine is likely to produce a transient “epinephrine response” within 45 seconds, consisting of an increase in heart rate and systolic blood pressure, circumoral pallor, palpitations and nervousness in the unsedated patient. The sedated patient may exhibit only a pulse rate increase of 20 or more beats per minute for 15 or more seconds. Patients on beta blockers may not manifest changes in heart rate, but blood pressure monitoring can detect an evanescent rise in systolic blood pressure. Adequate time should be allowed for onset of anesthesia after administration of each test dose. The rapid injection of a large volume of lidocaine hydrochloride injection through the catheter should be avoided, and, when feasible, fractional doses should be administered.
In the event of the known injection of a large volume of local anesthetic solution into the subarachnoid space, after suitable resuscitation and if the catheter is in place, consider attempting the recovery of drug by draining a moderate amount of cerebrospinal fluid (such as 10 mL) through the epidural catheter. -
MAXIMUM RECOMMENDED DOSAGES
Adults
For normal healthy adults, the maximum individual dose should not exceed 4.5 mg/kg (2 mg/lb) of body weight, and in general it is recom mended that the max i m um t otal dose does not exceed 300 mg. For continuous epidural or caudal anesthesia, the maximum recommended dosage should not be administered at intervals of less than 90 minutes. When continuous lumbar or caudal epidural anesthesia is used for non-obstetrical procedures, more drug may be administered if required to produce adequate anesthesia.
The maximum recommended dose per 90 minute period of lidocaine hydrochloride for paracervical block in obstetrical patients and non-obstetrical patients is 200 mg total. One half of the total dose is usually administered to each side. Inject slowly, five minutes between sides (see also discussion of paracervical block in PRECAUTIONS).
For intravenous regional anesthesia, the dose administered should not exceed 4 mg/kg in adults.Children
It is difficult to recommend a maximum dose of any drug for children, since this varies as a function of age and weight. For children over 3 years of age who have a normal lean body mass and normal body development, the maximum dose is determined by the child’s age and weight. For example, in a child of 5 years weighing 50 lbs the dose of lidocaine hydrochloride should not exceed 75 to 100 mg (1.5 to 2 mg/lb). The use of even more dilute solutions (i.e., 0.25 to 0.5%) and total dosages not to exceed 3 mg/kg (1.4 mg/lb) are recommended for induction of intravenous regional anesthesia in children.
In order to guard against systemic toxicity, the lowest effective concentration and lowest effective dose should be used at all times. In some cases it will be necessary to dilute available concentrations with 0.9% sodium chloride injection in order to obtain the required final concentration.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. Do not use if solution is discolored or contains a precipitate.Table 1: Recommended Dosages Procedure Lidocaine Hydrochloride Injection
(without epinephrine)Conc (%) Vol (mL) Total Dose (mg) *Dose determined by number of dermatomes to be anesthetized (2 to 3 mL/dermatome).
Infiltration
Percutaneous
0.5 or 1
1 to 60
5 to 300
Intravenous regional
0.5
10 to 60
50 to 300
Peripheral Nerve Blocks, e.g.,
Brachial
1.5
15 to 20
225 to 300
Dental
2
1 to 5
20 to 100
Intercostal
1
3
30
Paravertebral
1
3 to 5
30 to 50
Pudendal (each side)
1
10
100
Paracervical
Obstetrical analgesia (each side)
1
10
100
Sympathetic Nerve Blocks, e.g.,
Cervical (stellate ganglion)
1
5
50
Lumbar
1
5 to 10
50 to 100
Central Neural Blocks
Epidural*
Thoracic
1
20 to 30
200 to 300
Lumbar
Analgesia
1
25 to 30
250 to 300
Anesthesia
1.5
15 to 20
225 to 300
2
10 to 15
200 to 300
Caudal
Obstetrical analgesia
1
20 to 30
200 to 300
Surgical anesthesia
1.5
15 to 20
225 to 300
THE ABOVE SUGGESTED CONCENTRATIONS AND VOLUMES SERVE ONLY AS A GUIDE. OTHER VOLUMES AND CONCENTRATIONS MAY BE USED PROVIDED THE TOTAL MAXIMUM RECOMMENDED DOSE IS NOT EXCEEDED.
- STERILIZATION, STORAGE AND TECHNICAL PROCEDURES
-
HOW SUPPLIED
Lidocaine Hydrochloride Injection, USP is supplied as follows:
Lidocaine Hydrochloride Injection USP, 2% (20 mg/mL)
2 mL Single Dose Vials in a Carton of 10 NDC 55150-164-02
Sterile, Nonpyrogenic
Discard unused portion
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
The vial stopper is not made with natural rubber latex.
Distributed by:
AuroMedics Pharma LLC279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma LimitedHyderabad - 500038
India
Revised: February 2020 - SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Triamcinolone acetonide injectable suspension, USP is a synthetic glucocorticoid corticosteroid with anti-inflammatory action. THIS FORMULATION IS SUITABLE FOR INTRAMUSCULAR AND INTRA-ARTICULAR USE ONLY. THIS FORMULATION IS NOT FOR INTRADERMAL INJECTION.
Each mL of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, USP, with 0.65% sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose sodium, and 0.04% polysorbate 80 in an aqueous suspension. Sodium hydroxide or hydrochloric acid may be present to adjust pH to 5.0 to 7.5. At the time of manufacture, the air in the container is replaced by nitrogen.
The chemical name for triamcinolone acetonide is 9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone. Its structural formula is:
MW 434.50
Triamcinolone acetonide, USP occurs as a white to cream-colored, crystalline powder having not more than a slight odor and is practically insoluble in water and very soluble in alcohol.
-
CLINICAL PHARMACOLOGY
Glucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract.
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Synthetic analogs such as triamcinolone are primarily used for their anti-inflammatory effects in disorders of many organ systems.
Triamcinolone acetonide injectable suspension has an extended duration of effect which may be sustained over a period of several weeks. Studies indicate that following a single intramuscular dose of 60 mg to 100 mg of triamcinolone acetonide, adrenal suppression occurs within 24 to 48 hours and then gradually returns to normal, usually in 30 to 40 days. This finding correlates closely with the extended duration of therapeutic action achieved with the drug.
-
INDICATIONS AND USAGE
Intramuscular
Where oral therapy is not feasible, injectable corticosteroid therapy, including triamcinolone acetonide injectable suspension is indicated for intramuscularuseas follows:
Allergic states:Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity reactions, perennial or seasonal allergic rhinitis, serum sickness, transfusion reactions.
Dermatologic diseases:Bullous dermatitis herpetiformis, exfoliative erythroderma, mycosis fungoides, pemphigus, severe erythema multiforme (Stevens-Johnson syndrome).
Endocrine disorders:Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy, mineralocorticoid supplementation is of particular importance), congenital adrenal hyperplasia, hypercalcemia associated with cancer, nonsuppurative thyroiditis.
Gastrointestinal diseases:To tide the patient over a critical period of the disease in regional enteritis and ulcerative colitis.
Hematologic disorders:Acquired (autoimmune) hemolytic anemia, Diamond-Blackfan anemia, pure red cell aplasia, selected cases of secondary thrombocytopenia.
Miscellaneous:Trichinosis with neurologic or myocardial involvement, tuberculous meningitis with subarachnoid block or impending block when used with appropriate antituberculous chemotherapy.
Neoplastic diseases:For the palliative management of leukemias and lymphomas.
Nervous system:Acute exacerbations of multiple sclerosis; cerebral edema associated with primary or metastatic brain tumor or craniotomy.
Ophthalmic diseases:Sympathetic ophthalmia, temporal arteritis, uveitis and ocular inflammatory conditions unresponsive to topical corticosteroids.
Renal diseases:To induce diuresis or remission of proteinuria in idiopathic nephrotic syndrome or that due to lupus erythematosus.
Respiratory diseases:Berylliosis, fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy, idiopathic eosinophilic pneumonias, symptomatic sarcoidosis.
Rheumatic disorders:As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in acute gouty arthritis; acute rheumatic carditis; ankylosing spondylitis; psoriatic arthritis; rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy). For the treatment of dermatomyositis, polymyositis and systemic lupus erythematosus.
Intra-Articular
The intra-articular or soft tissue administrationof triamcinolone acetonide injectable suspension is indicated as adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in acute gouty arthritis, acute and subacute bursitis, acute nonspecific tenosynovitis, epicondylitis, rheumatoid arthritis, synovitis of osteoarthritis.
-
CONTRAINDICATIONS
Triamcinolone acetonide injectable suspension is contraindicated in patients who are hypersensitive to any components of this product (see WARNINGS: General).
Intramuscular corticosteroid preparations are contraindicated for idiopathic thrombocytopenic purpura.
-
WARNINGS
Serious Neurologic Adverse Reactions with Epidural Administration
Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids (see WARNINGS: Neurologic). Specific events reported include, but are not limited to, spinal cord infarction, paraplegia, quadriplegia, cortical blindness, and stroke. These serious neurologic events have been reported with and without use of fluoroscopy. The safety and effectiveness of epidural administration of corticosteroids have not been established, and corticosteroids are not approved for this use.
General
Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to that received in flush solutions containing benzyl alcohol. Administration of high dosages of medications containing this preservative must take into account the total amount of benzyl alcohol administered. The amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources (see PRECAUTIONS: Pediatric Use).
Rare instances of anaphylaxis have occurred in patients receiving corticosteroid therapy (see ADVERSE REACTIONS). Cases of serious anaphylaxis, including death, have been reported in individuals receiving triamcinolone acetonide injection, regardless of the route of administration.
Because triamcinolone acetonide injectable suspension is a suspension, it should notbe administered intravenously.
Unless a deepintramuscular injection is given, local atrophy is likely to occur (for recommendations on injection techniques, see DOSAGE AND ADMINISTRATION). Due to the significantly higher incidence of local atrophy when the material is injected into the deltoid area, this injection site should be avoided in favor of the gluteal area.
Increased dosage of rapidly acting corticosteroids is indicated in patients on corticosteroid therapy subjected to any unusual stress before, during, and after the stressful situation. Triamcinolone acetonide injectable suspension is a long-acting preparation, and is not suitable for use in acute stress situations. To avoid drug-induced adrenal insufficiency, supportive dosage may be required in times of stress (such as trauma, surgery, or severe illness) both during treatment with triamcinolone acetonide injectable suspension and for a year afterwards.
Results from one multicenter, randomized, placebo-controlled study with methylprednisolone hemisuccinate, an intravenous corticosteroid, showed an increase in early (at 2 weeks) and late (at 6 months) mortality in patients with cranial trauma who were determined not to have other clear indications for corticosteroid treatment. High doses of systemic corticosteroids, including triamcinolone acetonide injectable suspension should not be used for the treatment of traumatic brain injury.
Cardio-Renal
Average and large doses of corticosteroids can cause elevation of blood pressure, salt and water retention and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when they are used in large doses. Dietary salt restriction and potassium supplementation may be necessary (see PRECAUTIONS). All corticosteroids increase calcium excretion.
Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Endocrine
Corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment.
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage.
Infections
General
Patients who are on corticosteroids are more susceptible to infections than are healthy individuals. There may be decreased resistance and inability to localize infection when corticosteroids are used. Infection with any pathogen (viral, bacterial, fungal, protozoan, or helminthic) in any location of the body may be associated with the use of corticosteroids alone or in combination with other immunosuppressive agents. These infections may be mild to severe. With increasing doses of corticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may also mask some signs of current infection.
Fungal Infections
Corticosteroids may exacerbate systemic fungal infections and therefore should not be used in the presence of such infections unless they are needed to control drug reactions. There have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure (see PRECAUTIONS: Drug Interactions:Amphotericin B injection and potassium-depleting agents).
Special Pathogens
Latent disease may be activated or there may be an exacerbation of intercurrent infections due to pathogens, including those caused by Amoeba, Candida, Cryptococcus, Mycobacterium, Nocardia, Pneumocystis,or Toxoplasma.
It is recommended that latent amebiasis or active amebiasis be ruled out before initiating corticosteroid therapy in any patient who has spent time in the tropics or in any patient with unexplained diarrhea.
Similarly, corticosteroids should be used with great care in patients with known or suspected Strongyloides(threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloideshyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Corticosteroids should not be used in cerebral malaria.
Tuberculosis
The use of corticosteroids in patients with active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate anti-tuberculosis regimen. If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Vaccination
Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered. However, the response to such vaccines cannot be predicted.Immunization procedures may be undertaken in patients who are receiving corticosteroids as replacement therapy, e.g., for Addison’s disease.
Viral Infections
Chicken pox and measles can have a more serious or even fatal course in pediatric and adult patients on corticosteroids. In pediatric and adult patients who have not had these diseases, particular care should be taken to avoid exposure. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chicken pox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with immunoglobulin (IG) may be indicated (see the respective package inserts for complete VZIG and IG prescribing information). If chicken pox develops, treatment with antiviral agents should be considered.
Neurologic
Epidural and intrathecal administration of this product is not recommended. Reports of serious medical events, including death, have been associated with epidural and intrathecal routes of corticosteroid administration (see ADVERSE REACTIONS:Gastrointestinaland Neurologic/Psychiatric).
Ophthalmic
Use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. The use of oral corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Corticosteroids should not be used in active ocular herpes simplex.
Adequate studies to demonstrate the safety of triamcinolone acetonide injectable suspension use by intraturbinal, subconjunctival, sub-Tenons, retrobulbar and intraocular (intravitreal) injections have not been performed. Endophthalmitis, eye inflammation, increased intraocular pressure and visual disturbances including vision loss have been reported with intravitreal administration. Administration of triamcinolone acetonide injectable suspension intraocularly or into the nasal turbinates is not recommended.
Intraocular injection of corticosteroid formulations containing benzyl alcohol, such as triamcinolone acetonide injectable suspension is not recommended because of potential toxicity from the benzyl alcohol.
-
PRECAUTIONS
General
This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial.
The lowest possible dose of corticosteroid should be used to control the condition under treatment. When reduction in dosage is possible, the reduction should be gradual.
Since complications of treatment with glucocorticoids are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement.
Cardio-Renal
As sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids, these agents should be used with caution in patients with congestive heart failure, hypertension, or renal insufficiency.
Endocrine
Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
Gastrointestinal
Steroids should be used with caution in active or latent peptic ulcers, diverticulitis, fresh intestinal anastomoses and nonspecific ulcerative colitis, since they may increase the risk of a perforation.
Signs of peritoneal irritation following gastrointestinal perforation in patients receiving corticosteroids may be minimal or absent.
There is an enhanced effect of corticosteroids in patients with cirrhosis.
Intra-Articular and Soft Tissue Administration
Intra-articularly injected corticosteroids may be systemically absorbed.
Appropriate examination of any joint fluid present is necessary to exclude a septic process.
A marked increase in pain accompanied by local swelling, further restriction of joint motion, fever and malaise are suggestive of septic arthritis. If this complication occurs and the diagnosis of sepsis is confirmed, appropriate antimicrobial therapy should be instituted.
Injection of a steroid into an infected site is to be avoided. Local injection of a steroid into a previously infected joint is not usually recommended.
Corticosteroid injection into unstable joints is generally not recommended.
Intra-articular injection may result in damage to joint tissues (see ADVERSE REACTIONS:Musculoskeletal).
Musculoskeletal
Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (i.e. decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolism, and reduced sex hormone production, may lead to inhibition of bone growth in pediatric patients and the development of osteoporosis at any age. Special consideration should be given to patients at increased risk of osteoporosis (i.e. postmenopausal women) before initiating corticosteroid therapy.
Neuro-Psychiatric
Although controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that they affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect (see DOSAGE AND ADMINISTRATION).
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Psychiatric derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes and severe depression to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Ophthalmic
Intraocular pressure may become elevated in some individuals. If steroid therapy is continued for more than 6 weeks, intraocular pressure should be monitored.
Information for Patients
Patients should be warned not to discontinue the use of corticosteroids abruptly or without medical supervision, to advise any medical attendants that they are taking corticosteroids, and to seek medical advice at once should they develop fever or other signs of infection.
Persons who are on corticosteroids should be warned to avoid exposure to chicken pox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
Drug Interactions
Aminoglutethimide:Aminoglutethimide may lead to a loss of corticosteroid-induced adrenal suppression.
Amphotericin B injection and potassium-depleting agents:When corticosteroids are administered concomitantly with potassium-depleting agents (i.e. amphotericin B, diuretics), patients should be observed closely for development of hypokalemia. There have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure.
Antibiotics:Macrolide antibiotics have been reported to cause a significant decrease in corticosteroid clearance.
Anticholinesterases:Concomitant use of anticholinesterase agents and corticosteroids may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy.
Anticoagulants, oral:Co-administration of corticosteroids and warfarin usually results in inhibition of response to warfarin, although there have been some conflicting reports. Therefore, coagulation indices should be monitored frequently to maintain the desired anticoagulant effect.
Antidiabetics:Because corticosteroids may increase blood glucose concentrations, dosage adjustments of antidiabetic agents may be required.
Antitubercular drugs:Serum concentrations of isoniazid may be decreased.
Cholestyramine:Cholestyramine may increase the clearance of corticosteroids.
Cyclosporine:Increased activity of both cyclosporine and corticosteroids may occur when the two are used concurrently. Convulsions have been reported with this concurrent use.
CYP 3A4 inhibitors:Triamcinolone acetonide is a substrate of CYP3A4. Ketoconazole has been reported to decrease the metabolism of certain corticosteroids by up to 60%, leading to an increased risk of corticosteroid side effects. Co-administration of other strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin, cobicistat-containing products) with triamcinolone acetonide injectable suspension may cause increased plasma concentration of triamcinolone leading to adverse reactions (see ADVERSE REACTIONS). During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving triamcinolone acetonide and strong CYP3A4 inhibitors (e.g., ritonavir) (see WARNINGS, Endocrineand PRECAUTIONS, Endocrine). Consider the benefit-risk of concomitant use and monitor for systemic corticosteroid side effects.
Digitalis glycosides:Patients on digitalis glycosides may be at increased risk of arrhythmias due to hypokalemia.
Estrogens, including oral contraceptives:Estrogens may decrease the hepatic metabolism of certain corticosteroids, thereby increasing their effect.
Hepatic enzyme inducers (e.g., barbiturates, phenytoin, carbamazepine, rifampin):Drugs which induce hepatic microsomal drug metabolizing enzyme activity may enhance the metabolism of corticosteroids and require that the dosage of the corticosteroid be increased.
Nonsteroidal anti-inflammatory drugs (NSAIDs):Concomitant use of aspirin (or other nonsteroidal anti-inflammatory drugs) and corticosteroids increases the risk of gastrointestinal side effects. Aspirin should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia. The clearance of salicylates may be increased with concurrent use of corticosteroids.
Skin tests:Corticosteroids may suppress reactions to skin tests.
Vaccines:Patients on prolonged corticosteroid therapy may exhibit a diminished response to toxoids and live or inactivated vaccines due to inhibition of antibody response. Corticosteroids may also potentiate the replication of some organisms contained in live attenuated vaccines. Routine administration of vaccines or toxoids should be deferred until corticosteroid therapy is discontinued if possible (see WARNINGS: Infections: Vaccination).
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether corticosteroids have a potential for carcinogenesis or mutagenesis.
Steroids may increase or decrease motility and number of spermatozoa in some patients.
Pregnancy
Teratogenic Effects
Corticosteroids have been shown to be teratogenic in many species when given in doses equivalent to the human dose. Animal studies in which corticosteroids have been given to pregnant mice, rats and rabbits have yielded an increased incidence of cleft palate in the offspring. There are no adequate and well-controlled studies in pregnant women. Corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when corticosteroids are administered to a nursing woman.
Pediatric Use
This product contains benzyl alcohol as a preservative. Benzyl alcohol, a component of this product, has been associated with serious adverse events and death, particularly in pediatric patients. The “gasping syndrome” (characterized by central nervous system depression, metabolic acidosis, gasping respirations and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages > 99 mg/kg/day in neonates and low-birth-weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia and cardiovascular collapse. Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the “gasping syndrome,” the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birth-weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
The efficacy and safety of corticosteroids in the pediatric population are based on the well-established course of effect of corticosteroids which is similar in pediatric and adult populations. Published studies provide evidence of efficacy and safety in pediatric patients for the treatment of nephrotic syndrome (> 2 years of age), and aggressive lymphomas and leukemias (> 1 month of age). Other indications for pediatric use of corticosteroids, e.g., severe asthma and wheezing, are based on adequate and well-controlled trials conducted in adults, on the premises that the course of the diseases and their pathophysiology are considered to be substantially similar in both populations.
The adverse effects of corticosteroids in pediatric patients are similar to those in adults (see ADVERSE REACTIONS). Like adults, pediatric patients should be carefully observed with frequent measurements of blood pressure, weight, height, intraocular pressure and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts and osteoporosis. Pediatric patients who are treated with corticosteroids by any route, including systemically administered corticosteroids, may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been observed at low systemic doses and in the absence of laboratory evidence of HPA axis suppression (i.e. cosyntropin stimulation and basal cortisol plasma levels). Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The linear growth of pediatric patients treated with corticosteroids should be monitored, and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of treatment alternatives. In order to minimize the potential growth effects of corticosteroids, pediatric patients should be titratedto the lowest effective dose.
Geriatric Use
No overall differences in safety or effectiveness were observed between elderly subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
ADVERSE REACTIONS
(listed alphabetically under each subsection)
The following adverse reactions may be associated with corticosteroid therapy:
Allergic reactions:Anaphylaxis including death, angioedema.
Cardiovascular:Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction (see WARNINGS), pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis.
Dermatologic:Acne, allergic dermatitis, cutaneous and subcutaneous atrophy, dry scaly skin, ecchymoses and petechiae, edema, erythema, hyperpigmentation, hypopigmentation, impaired wound healing, increased sweating, lupus erythematosus-like lesions, purpura, rash, sterile abscess, striae, suppressed reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria.
Endocrine:Decreased carbohydrate and glucose tolerance, development of cushingoid state, glycosuria, hirsutism, hypertrichosis, increased requirements for insulin or oral hypoglycemic agents in diabetes, manifestations of latent diabetes mellitus, menstrual irregularities, postmenopausal vaginal hemorrhage, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery, or illness), suppression of growth in pediatric patients.
Fluid and electrolyte disturbances:Congestive heart failure in susceptible patients, fluid retention, hypokalemic alkalosis, potassium loss, sodium retention.
Gastrointestinal:Abdominal distention, bowel/bladder dysfunction (after intrathecal administration [see WARNINGS: Neurologic]), elevation in serum liver enzyme levels (usually reversible upon discontinuation), hepatomegaly, increased appetite, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, perforation of the small and large intestine (particularly in patients with inflammatory bowel disease), ulcerative esophagitis.
Metabolic:Negative nitrogen balance due to protein catabolism.
Musculoskeletal:Aseptic necrosis of femoral and humeral heads, calcinosis (following intra-articular or intralesional use), Charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, post injection flare (following intra-articular use), steroid myopathy, tendon rupture, vertebral compression fractures.
Neurologic/Psychiatric:Convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudotumor cerebri) usually following discontinuation of treatment, insomnia, mood swings, neuritis, neuropathy, paresthesia, personality changes, psychiatric disorders, vertigo. Arachnoiditis, meningitis, paraparesis/paraplegia and sensory disturbances have occurred after intrathecal administration. Spinal cord infarction, paraplegia, quadriplegia, cortical blindness and stroke (including brainstem) have been reported after epidural administration of corticosteroids (see WARNINGS: Serious Neurologic Adverse Reactions with Epidural Administrationand WARNINGS: Neurologic).
Ophthalmic:Exophthalmos, glaucoma, increased intraocular pressure, posterior subcapsular cataracts, rare instances of blindness associated with periocular injections.
Other:Abnormal fat deposits, decreased resistance to infection, hiccups, increased or decreased motility and number of spermatozoa, malaise, moon face, weight gain.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
General
NOTE: CONTAINS BENZYL ALCOHOL (see PRECAUTIONS).
The initial dose of triamcinolone acetonide injectable suspension may vary from 2.5 mg to 100 mg per day depending on the specific disease entity being treated (see Dosagesection below). However, in certain overwhelming, acute, life-threatening situations, administration in dosages exceeding the usual dosages may be justified and may be in multiples of the oral dosages.
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT.After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. Situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient’s individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment. In this latter situation it may be necessary to increase the dosage of the corticosteroid for a period of time consistent with the patient’s condition. If after long-term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
Dosage
SYSTEMIC
The suggested initial dose is 60 mg, injected deeply into the gluteal muscle. Atrophy of subcutaneous fat may occur if the injection is not properly given. Dosage is usually adjusted within the range of 40 mg to 80 mg, depending upon patient response and duration of relief. However, some patients may be well controlled on doses as low as 20 mg or less.
Hay fever or pollen asthma: Patients with hay fever or pollen asthma who are not responding to pollen administration and other conventional therapy may obtain a remission of symptoms lasting throughout the pollen season after a single injection of 40 mg to 100 mg.
In the treatment of acute exacerbations of multiple sclerosis, daily doses of 160 mg of triamcinolone for a week followed by 64 mg every other day for one month are recommended (see PRECAUTIONS: Neuro-Psychiatric).
In pediatric patients, the initial dose of triamcinolone may vary depending on the specific disease entity being treated. The range of initial doses is 0.11 to 1.6 mg/kg/day in 3 or 4 divided doses (3.2 to 48 mg/m 2bsa/day).
For the purpose of comparison, the following is the equivalent milligram dosage of the various glucocorticoids:
Cortisone, 25
Triamcinolone, 4
Hydrocortisone, 20
Paramethasone, 2
Prednisolone, 5
Betamethasone, 0.75
Prednisone, 5
Dexamethasone, 0.75
Methylprednisolone, 4
These dose relationships apply only to oral or intravenous administration of these compounds. When these substances or their derivatives are injected intramuscularly or into joint spaces, their relative properties may be greatly altered.
LOCAL
Intra-articular administration:A single local injection of triamcinolone acetonide is frequently sufficient, but several injections may be needed for adequate relief of symptoms.
Initial dose:2.5 mg to 5 mg for smaller joints and from 5 mg to 15 mg for larger joints, depending on the specific disease entity being treated. For adults, doses up to 10 mg for smaller areas and up to 40 mg for larger areas have usually been sufficient. Single injections into several joints, up to a total of 80 mg, have been given.
Administration
GENERAL
STRICT ASEPTIC TECHNIQUE IS MANDATORY.The vial should be shaken before use to ensure a uniform suspension. Prior to withdrawal, the suspension should be inspected for clumping or granular appearance (agglomeration). An agglomerated product results from exposure to freezing temperatures and should not be used. After withdrawal, triamcinolone acetonide injectable suspension should be injected without delay to prevent settling in the syringe. Careful technique should be employed to avoid the possibility of entering a blood vessel or introducing infection.
SYSTEMIC
For systemic therapy, injection should be made deeply into the gluteal muscle(see WARNINGS). For adults, a minimum needle length of 1½ inches is recommended. In obese patients, a longer needle may be required. Use alternative sites for subsequent injections.
LOCAL
For treatment of joints, the usual intra-articular injection technique should be followed. If an excessive amount of synovial fluid is present in the joint, some, but not all, should be aspirated to aid in the relief of pain and to prevent undue dilution of the steroid.
With intra-articular administration, prior use of a local anesthetic may often be desirable. Care should be taken with this kind of injection, particularly in the deltoid region, to avoid injecting the suspension into the tissues surrounding the site, since this may lead to tissue atrophy.
In treating acute nonspecific tenosynovitis, care should be taken to ensure that the injection of the corticosteroid is made into the tendon sheath rather than the tendon substance. Epicondylitis may be treated by infiltrating the preparation into the area of greatest tenderness.
-
HOW SUPPLIED
Triamcinolone Acetonide Injectable Suspension, USP is supplied in vials providing 40 mg triamcinolone acetonide per mL.
40 mg/mL, 1 mL
1 mL single-dose vial: NDC 70121-1049-1
25 single-dose vials in 1 carton: NDC 70121-1049-5
Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].Protect from temperatures below 20°C (68°F). Store vial in carton to protect from light. Store vial upright.
Discard unused portions of single-dose vials.
Manufactured by:
Amneal Pharmaceuticals Pvt. Ltd.
Parenteral Unit
Ahmedabad 382213, INDIA
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 08-2020-05
- Povidone Iodine Swabsticks
- ACTIVE INGREDIENT
- Purpose:
- Warnings:
- Do not use:
- Ask a doctor before use if you have:
- Stop Use:
- Keep Out Of Reach Of Children
- Directions Povidone iodine:
- Other information:
- INDICATIONS & USAGE
- Inactive Ingredients
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 76420-740-01
Rx Only
MLK F1 Kit™
Kit Contains
1 Bupivacaine HCl 0.5% Single Dose Vial (10mL)
1 Lidocaine HCl Injection, USP 2% (2mL)
2 Triamcinolone Acetonide Injectable Suspension, USP 40 mg/mL (1 mL)
1 Povidone-Iodine Swabsticks (3 Swabs)
1 Pair Nitrile Powder Free Sterile Gloves (M)
1 Drape
1 Adhesive Bandage
5 Non Sterile 4x4 Gauze
Needles and Syringes Not Included
1 Dose
Single Use Only
Distributed by
Enovachem™
PHARMACEUTICALS
Torrance, CA 90501
-
INGREDIENTS AND APPEARANCE
MLK F1 KIT
marcaine, lidocaine, kenalog, povidone iodine kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76420-740 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76420-740-01 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 05/23/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 10 mL Part 2 1 VIAL, SINGLE-DOSE 2 mL Part 3 2 VIAL 2 mL Part 4 1 PACKET 0.9 mL Part 1 of 4 BUPIVACAINE HYDROCHLORIDE
bupivacaine hydrochloride injection, solutionProduct Information Item Code (Source) NDC:0409-1162 Route of Administration EPIDURAL, INTRACAUDAL, PERINEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE (UNII: 7TQO7W3VT8) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.1 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-1162-18 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070584 11/22/2005 Part 2 of 4 LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride injection, solutionProduct Information Item Code (Source) NDC:55150-164 Route of Administration EPIDURAL, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 6 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55150-164-02 10 in 1 CARTON 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203082 03/14/2013 Part 3 of 4 TRIAMCINOLONE ACETONIDE
triamcinolone acetonide injection, suspensionProduct Information Item Code (Source) NDC:70121-1049 Route of Administration INTRAMUSCULAR, INTRA-ARTICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAMCINOLONE ACETONIDE (UNII: F446C597KA) (TRIAMCINOLONE ACETONIDE - UNII:F446C597KA) TRIAMCINOLONE ACETONIDE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Product Characteristics Color white (white to cream-color) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70121-1049-5 25 in 1 CARTON 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207550 12/11/2017 Part 4 of 4 POVIDINE IODINE
povidine iodine swabProduct Information Item Code (Source) NDC:67777-419 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) CITRIC ACID ACETATE (UNII: DSO12WL7AU) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67777-419-02 0.9 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 09/13/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/16/2014 Labeler - Asclemed USA, Inc. (059888437)

