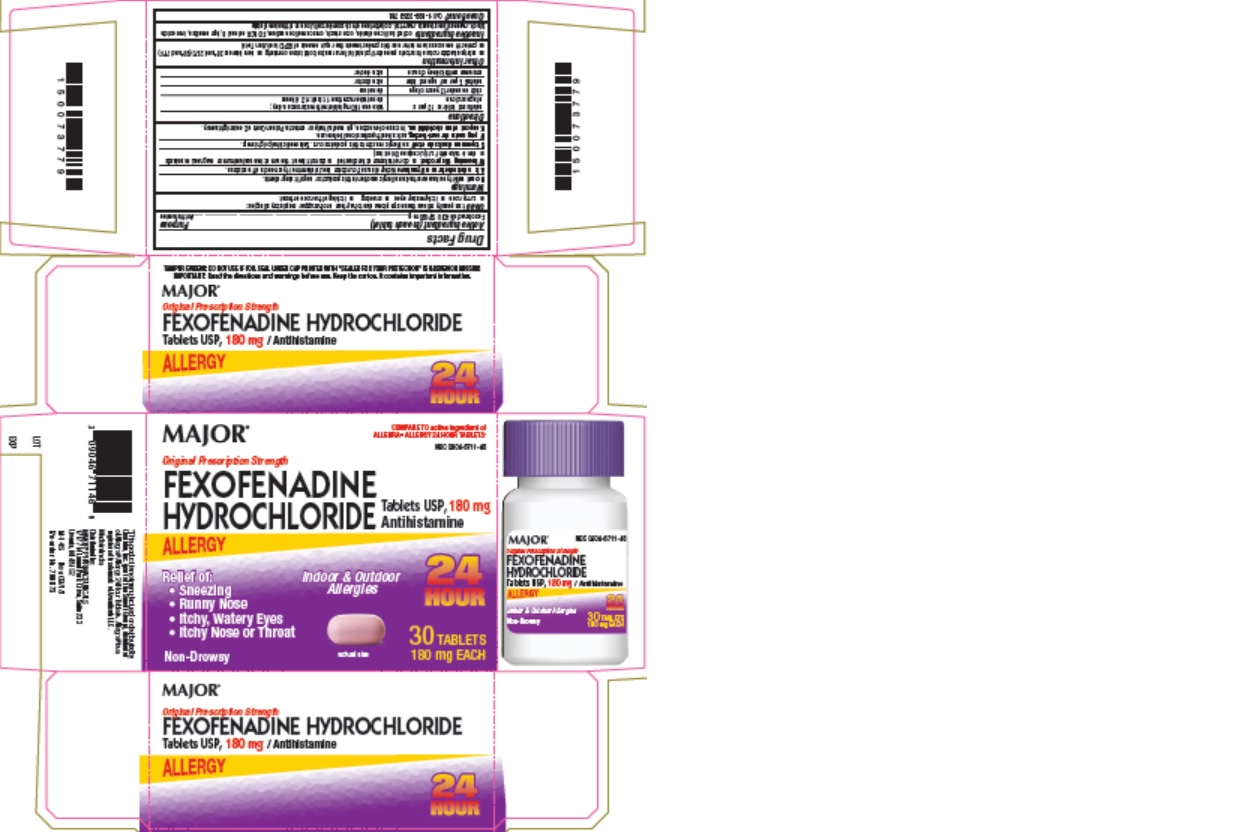
Label: FEXOFENADINE HYDROCHLORIDE tablet
-
NDC Code(s):
0904-6711-10,
0904-6711-46,
0904-6711-52,
0904-6711-89, view more0904-6711-92
- Packager: MAJOR PHARMACEUTICALS
- This is a repackaged label.
- Source NDC Code(s): 55111-784
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Package Label - 30 Count Carton
MAJOR
COMPARE TO active ingredient of
ALLEGRA® ALLERGY 24 HOUR TABLETS*NDC 0904-6711-46
Original Prescription Strength
FEXOFENADINE
HYDROCHLORIDE Tablets USP, 180 mg
AntihistamineALLERGY
Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat
Non-Drowsy
Indoor & Outdoor
Allergies24
HOUR30 TABLETS
180 mg EACH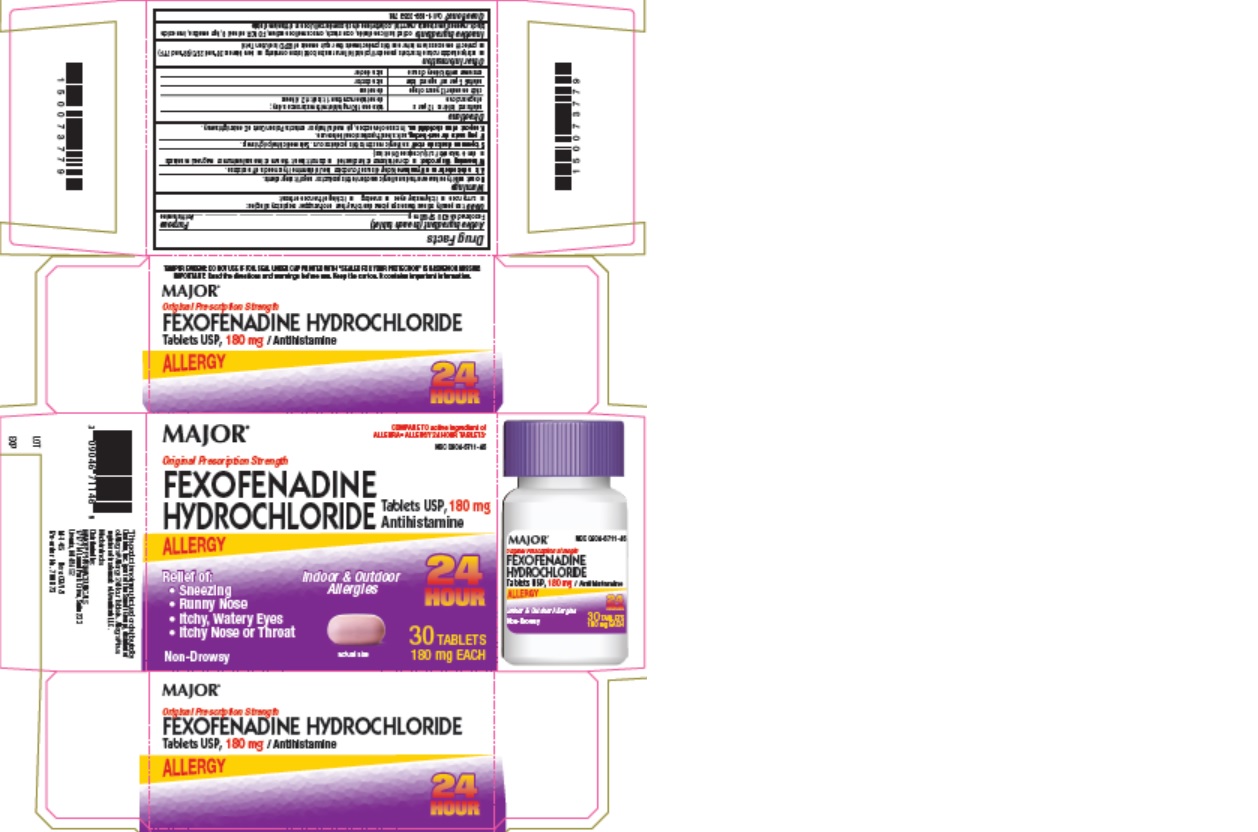
- Package Label - 30 Count Bottle
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6711(NDC:55111-784) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fexofenadine Hydrochloride (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) Fexofenadine Hydrochloride 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) magnesium stearate (UNII: 70097M6I30) mannitol (UNII: 3OWL53L36A) POWDERED CELLULOSE (UNII: SMD1X3XO9M) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) FERROSOFERRIC OXIDE (UNII: XM0M87F357) polyethylene glycol 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK Score no score Shape OVAL Size 7mm Flavor Imprint Code 194;R Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6711-46 1 in 1 CARTON 04/27/2018 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0904-6711-52 2 in 1 CARTON 04/27/2018 10/30/2022 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:0904-6711-10 3 in 1 CARTON 04/27/2018 10/30/2022 3 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:0904-6711-89 1 in 1 CARTON 04/27/2018 10/30/2022 4 90 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:0904-6711-92 1 in 1 CARTON 04/27/2018 10/30/2022 5 150 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076502 04/27/2018 Labeler - MAJOR PHARMACEUTICALS (191427277)