Label: RETIN-A MICRO- tretinoin gel
-
NDC Code(s):
0187-5140-20,
0187-5140-45,
0187-5140-50,
0187-5144-20, view more0187-5144-45, 0187-5144-50, 0187-5146-02, 0187-5146-50, 0187-5148-02, 0187-5148-50
- Packager: Bausch Health US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RETIN-A MICRO safely and effectively. See full prescribing information for RETIN-A MICRO.
RETIN-A MICRO ®(tretinoin) gel microsphere, 0.1%, 0.08%, 0.06% and 0.04%, for topical use
Initial U.S. Approval: 1971INDICATIONS AND USAGE
Retin-A Micro is a retinoid, indicated for topical treatment of acne vulgaris. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Gel, 0.1%, 0.08%, 0.06%, and 0.04% ( 3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Retin-A Micro should not be used on eczematous or sunburned skin due to potential for severe irritation. ( 5.1, 5.2)
- Avoid unprotected exposure to sunlight including sunlamps (UV light), when using Retin-A Micro due to potential for increased photosensitization. Use sunscreen of at least SPF 15 and protective clothing during exposure. ( 5.2)
- Avoid use of Retin-A Micro with weather extremes, such as wind or cold due to potential for increased irritation. ( 5.2)
ADVERSE REACTIONS
Most common adverse reactions are skin pain, pruritus, skin irritation/subcutaneous irritation, pharyngitis, and erythema. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Retin-A Micro should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. There are no adequate and well-controlled studies in pregnant and nursing women. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Irritation
5.2 Exposure to Ultraviolet Light or Weather Extremes
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Retin-A Micro (tretinoin) Gel microsphere, 0.1%
14.2 Retin-A Micro (tretinoin) Gel microsphere, 0.04%
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Storage Conditions
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For topical use only. Not for ophthalmic, oral, or intravaginal use.
Retin-A Micro should be applied once a day, in the evening, to the skin where acne lesions appear, using enough to cover the entire affected area in a thin layer. Areas to be treated should be cleansed thoroughly before the medication is applied. If medication is applied excessively, no more rapid or better results will be obtained and marked redness, peeling, or discomfort may occur. A transitory feeling of warmth or slight stinging may be noted on application. In cases where it has been necessary to temporarily discontinue therapy or to reduce the frequency of application, therapy may be resumed or the frequency of application increased as the patient becomes able to tolerate the treatment. Frequency of application should be closely monitored by careful observation of the clinical therapeutic response and skin tolerance. Efficacy has not been established for less than once daily dosing frequencies.
During the early weeks of therapy, an apparent exacerbation of inflammatory lesions may occur. If tolerated, this should not be considered a reason to discontinue therapy [ see ADVERSE REACTIONS( 6.1) ].
Therapeutic results may be noticed after two weeks, but more than seven weeks of therapy are required before consistent beneficial effects are observed.
Retin-A Micro should be kept away from the eyes, the mouth, paranasal creases of the nose, and mucous membranes.
Patients treated with Retin-A Micro may use cosmetics.
Concomitant topical medication, medicated or abrasive soaps and cleansers, products that have a strong drying effect, products with high concentrations of alcohol, astringents, or spices should be used with caution because of possible interaction with tretinoin. Avoid contact with the peel of limes. Particular caution should be exercised with the concomitant use of topical over-the-counter acne preparations containing benzoyl peroxide, sulfur, resorcinol, or salicylic acid with Retin-A Micro. It also is advisable to allow the effects of such preparations to subside before use of Retin-A Micro is begun.
-
3 DOSAGE FORMS AND STRENGTHS
Retin-A Micro is a white to very pale yellow opaque gel. Retin-A Micro is available in four strengths: 0.1%, 0.08%, 0.06% and 0.04%.
Each gram of Retin-A Micro Gel, 0.1%, contains 1 mg of tretinoin.
Each gram of Retin-A Micro Gel, 0.08%, contains 0.8 mg of tretinoin.
Each gram of Retin-A Micro Gel, 0.06%, contains 0.6 mg of tretinoin.
Each gram of Retin-A Micro Gel, 0.04%, contains 0.4 mg of tretinoin.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Local Irritation
The skin of certain individuals may become excessively dry, red, swollen, or blistered.
Tretinoin has been reported to cause severe irritation on eczematous skin and should be used with utmost caution in patients with this condition.
If the degree of irritation warrants, patients should be directed to temporarily reduce the amount or frequency of application of the medication, discontinue use temporarily, or discontinue use all together. Efficacy at reduced frequencies of application has not been established. If a reaction suggesting sensitivity occurs, use of the medication should be discontinued.
To help limit skin irritation, patients must:
- wash the treated skin gently, using a mild, non-medicated soap, and pat it dry, and
- avoid washing the treated skin too often or scrubbing it hard when washing.
Patients should apply a topical moisturizer if dryness is bothersome.
5.2 Exposure to Ultraviolet Light or Weather Extremes
Unprotected exposure to sunlight, including sunlamps (UV light) should be avoided or minimized during the use of Retin-A Micro and patients with sunburn should be advised not to use the product until fully recovered because of heightened susceptibility to sunlight as a result of the use of tretinoin. Patients who may be required to have extended periods of UV exposure (e.g., due to occupation or sports), or those with inherent sensitivity to the sun, or those using medications that cause photosensitivity, should exercise particular caution. Use of sunscreen products (SPF 15 or higher) and protective clothing over treated areas are recommended when exposure cannot be avoided [ see Nonclinical Toxicology (13.1)].
Weather extremes, such as wind or cold, also may be irritating to tretinoin-treated skin.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Subjects with Acne
In separate clinical trials for each concentration, acne subjects treated with Retin-A Micro (tretinoin) Gel microsphere, 0.1% or 0.04%, over the twelve-week period showed that cutaneous irritation scores for erythema, peeling, dryness, burning/stinging, or itching peaked during the initial two weeks of therapy, decreasing thereafter.
Approximately half of the subjects treated with Retin-A Micro, 0.04%, had cutaneous irritation at Week 2. Of those subjects who did experience cutaneous side effects, most had signs or symptoms that were mild in severity (severity was ranked on a 4-point ordinal scale: 0=none, 1=mild, 2=moderate, and 3=severe). Less than 10% of patients experienced moderate cutaneous irritation and there was no severe irritation at Week 2.
In trials of Retin-A Micro (tretinoin) Gel microsphere, 0.04%, throughout the treatment period the majority of subjects experienced some degree of irritation (mild, moderate, or severe) with 1% (2/225) of subjects having scores indicative of a severe irritation; 1.3% (3/225) of subjects treated with Retin-A Micro (tretinoin) Gel microsphere, 0.04%, discontinued treatment due to irritation, which included dryness in one patient and peeling and urticaria in another.
In trials of Retin-A Micro (tretinoin) Gel microsphere, 0.1%, no more than 3% of subjects had cutaneous irritation scores indicative of severe irritation; 6% (14/224) of subjects treated with Retin-A Micro (tretinoin) Gel microsphere, 0.1%, discontinued treatment due to irritation. Of these 14 subjects, four had severe irritation after 3 to 5 days of treatment, with blistering in one subject.
In a double-blind trial with 156 acne subjects comparing 12 weeks of treatment with Retin-A Micro (tretinoin) Gel, 0.04% or 0.1%, (78 subjects each group), the most frequently-reported adverse events affected the skin and subcutaneous tissue (15.4% in the 0.04% group, and 20.5% in the 0.1% group). The most prevalent of the dermatologic adverse events in the 0.04% group was skin irritation (6.4%); and in the 0.1% group skin burning (7.7%), erythema (5.1%), skin irritation (3.8%), and dermatitis (3.8%). Most adverse events were of mild intensity (63.4%), and 34.4% were moderate. One subject in each group had adverse events characterized as severe, neither were dermatologic findings and neither was characterized as related to drug by the investigator.
Trials in Subjects without Acne
In a half-face comparison trial conducted for up to 14 days in women with sensitive skin, but without acne, Retin-A Micro (tretinoin) Gel microsphere, 0.1%, was statistically less irritating than tretinoin cream, 0.1%. In addition, a cumulative 21- day irritation evaluation in subjects with normal skin showed that Retin-A Micro (tretinoin) Gel microsphere, 0.1%, had a lower irritation profile than tretinoin cream, 0.1%. The clinical significance of these irritation studies for patients with acne is not established. Comparable effectiveness of Retin-A Micro (tretinoin) Gel microsphere, 0.1%, and tretinoin cream, 0.1%, has not been established. The lower irritancy of Retin-A Micro (tretinoin) Gel microsphere, 0.1%, in subjects without acne may be attributable to the properties of its vehicle. The contribution of decreased irritancy by the MICROSPONGE ®System has not been established. No irritation trials have been performed to compare Retin-A Micro (tretinoin) Gel microsphere, 0.04%, with either Retin-A Micro (tretinoin) Gel microsphere, 0.1%, or tretinoin cream, 0.1%.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Retin-A Micro Gel. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Temporary hyper- or hypopigmentation has been reported with repeated application of tretinoin.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Retin-A Micro should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Thirty human cases of temporally associated congenital malformations have been reported during two decades of clinical use of tretinoin products. Although no definite pattern of teratogenicity and no causal association has been established from these cases, five of the reports describe the rare birth defect category holoprosencephaly (defects associated with incomplete midline development of the forebrain). The significance of these spontaneous reports in terms of risk to the fetus is not known.
For purposes of comparison of the animal exposure to systemic human exposure, the MRHD applied topically is defined as 1 gram of Retin-A Micro (tretinoin) Gel microsphere, 0.1%, applied daily to a 60 kg person (0.017 mg tretinoin/kg body weight).
Pregnant rats were treated with Retin-A Micro (tretinoin) Gel microsphere, 0.1%, at daily dermal doses of 0.5 to 1.0 mg/kg/day tretinoin on gestation days 6-15. Alterations were seen in vertebrae and ribs of offspring at 5 to 10 times the MRHD based on the body surface area (BSA) comparison.
Pregnant New Zealand White rabbits were treated with Retin-A Micro (tretinoin) Gel microsphere, 0.1%, at daily dermal doses of 0.2, 0.5, and 1.0 mg/kg/day tretinoin on gestation days 7-19. Doses were administered topically for 24 hours a day while wearing Elizabethan collars to prevent ingestion of the drug. Increased incidences of certain alterations, including domed head and hydrocephaly, typical of retinoid-induced fetal malformations in this species, were observed at 0.5 and 1.0 mg/kg/day. Similar malformations were not observed at 0.2 mg/kg/day, 4 times the MRHD based on BSA comparison. Other pregnant rabbits exposed topically for six hours per day to 0.5 or 1.0 mg/kg/day tretinoin while restrained in stocks to prevent ingestion, did not show any malformations at doses up to 19 times (1.0 mg/kg/day) the MRHD based on BSA comparison, but fetal resorptions were increased at 0.5 mg/kg (10 times the MRHD based on BSA comparison).
Oral tretinoin has been shown to cause malformations in rats, mice, rabbits, hamsters, and nonhuman primates.
Tretinoin induced fetal malformations in Wistar rats when given orally at doses greater than 1 mg/kg/day (10 times the MRHD based on BSA comparison). In the cynomolgus monkey, fetal malformations were reported for doses of 10 mg/kg/day but none were observed at 5 mg/kg/day (95 times the MRHD based on BSA comparison), although increased skeletal variations were observed at all doses. Dose-related increases in embryolethality and abortion also were reported. Similar results have also been reported in pigtail macaques.
In oral peri- and postnatal development studies in rats with tretinoin, decreased survival of neonates and growth retardation were observed at doses in excess of 2 mg/kg/day (19 times the MRHD based on BSA comparison).
Nonteratogenic effects on fetus
Oral tretinoin has been shown to be fetotoxic in rats when administered at doses 24 times the MRHD based on BSA comparison.
Topical tretinoin has been shown to be fetotoxic in rabbits when administered at doses 10 times the MRHD based on BSA comparison.
8.3 Nursing Mothers
It is not known whether tretinoin and/or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Retin-A Micro is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in children below the age of 12 have not been established.
8.5 Geriatric Use
Safety and effectiveness in a geriatric population have not been established. Clinical trials of Retin-A Micro (tretinoin) Gel microsphere, 0.1% and 0.04%, did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently from younger subjects.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Retin-A Micro (tretinoin) Gel microsphere, 0.1%, 0.08%, 0.06% and 0.04% is a white to very pale yellow opaque gel for topical treatment of acne vulgaris.
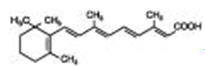
Chemically, tretinoin is all-trans-retinoic acid, also known as (all-E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid. It is a member of the retinoid class of compounds and a metabolite of naturally occurring Vitamin A. Tretinoin has a molecular weight of 300.44, a molecular formula of C 20H 28O 2and the following chemical structure:
Each gram of Retin-A Micro Gel, 0.1%, contains 1 mg of tretinoin.
Each gram of Retin-A Micro Gel, 0.08%, contains 0.8 mg of tretinoin.
Each gram of Retin-A Micro Gel, 0.06%, contains 0.6 mg of tretinoin.
Each gram of Retin-A Micro Gel, 0.04%, contains 0.4 mg of tretinoin.
The formulation contains methyl methacrylate/glycol dimethacrylate crosspolymer (MICROSPONGE ®System), propylene glycol dicaprylate/dicaprate and butylated hydroxytoluene to enable inclusion of the active ingredient, tretinoin, in an aqueous gel. Other components consist of benzyl alcohol, butylated hydroxytoluene, carbomer 974P, cyclomethicone and dimethicone copolyol, disodium EDTA, glycerin, PPG-20 methyl glucose ether distearate, propylene glycol, purified water, sorbic acid, trolamine, methyl ethacrylate/glycol dimethacrylate crosspolymer, propylene glycol dicaprylate/dicaprate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Although tretinoin activates three members of the retinoic acid (RAR) nuclear receptors (RARα, RARß, and RARγ) which may act to modify gene expression, subsequent protein synthesis, and epithelial cell growth and differentiation, it has not been established whether the clinical effects of tretinoin are mediated through activation of retinoic acid receptors and/or other mechanisms.
The exact mode of action of tretinoin is unknown. Current evidence suggests that topical tretinoin decreases cohesiveness of follicular epithelial cells with decreased microcomedone formation. Additionally, tretinoin stimulates mitotic activity and increased turnover of follicular epithelial cells causing extrusion of the comedones.
12.3 Pharmacokinetics
Tretinoin is a metabolite of Vitamin A metabolism in man. Percutaneous absorption, as determined by the cumulative excretion of radiolabeled drug into urine and feces, was assessed in 44 healthy men and women after single and repeated daily applications of 500 mg of a 0.1% tretinoin gel formulation. Estimates of in vivobioavailability, mean (SD)%, following both single and multiple daily applications, for a period of 28 days with the 0.1% gel, were 0.82 (0.11)% and 1.41 (0.54)%, respectively. The plasma concentrations of tretinoin and its metabolites, 13- cis-retinoic acid, all- trans-4-oxo-retinoic acid, and 13- cis-4-oxo-retinoic acid, generally ranged from 1 to 3 ng/mL and were essentially unaltered after either single or multiple daily applications of Retin-A Micro (tretinoin) Gel microsphere, 0.1%, relative to baseline levels. Clinical pharmacokinetic studies have not been performed with Retin-A Micro (tretinoin) Gel microsphere, 0.08%, 0.06% and 0.04%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dermal carcinogenicity testing has not been performed with Retin-A Micro (tretinoin) Gel microsphere, 0.1%, 0.08%, 0.06% or 0.04%.
In a 91-week dermal study in which CD-1 mice were administered 0.017% and 0.035% formulations of tretinoin, cutaneous squamous cell carcinomas and papillomas in the treatment area were observed in some female mice. These concentrations are near the tretinoin concentration of the 0.04% and 0.1% clinical formulations. A dose-related incidence of liver tumors in male mice was observed at those same doses. The maximum systemic doses associated with the administered 0.017% and 0.035% formulations are 0.5 and 1.0 mg/kg/day tretinoin, respectively. These doses are two and four times the MRHD based on BSA comparison.
The biological significance of these findings is not clear because they occurred at doses that exceeded the dermal maximally tolerated dose of tretinoin and because they were within the background natural occurrence rate for these tumors in this strain of mice.
There was no evidence of carcinogenic potential when 0.025 mg/kg/day of tretinoin was administered topically to mice (0.1 times the MRHD based on BSA comparison).
Studies in hairless albino mice suggest that concurrent exposure to tretinoin may enhance the tumorigenic potential of carcinogenic doses of UVB and UVA light from a solar simulator. This effect has been confirmed in a later study in pigmented mice, and dark pigmentation did not overcome the enhancement of photocarcinogenesis by 0.05% tretinoin. Although the significance of these studies to humans is not clear, patients should minimize exposure to sunlight or artificial ultraviolet irradiation sources [ see WARNINGS AND PRECAUTIONS( 5.2) ].
The genotoxic potential of tretinoin was evaluated in the Ames assay and in the in vivo mouse micronucleus assay, both of which were negative.
The components of the microspheres have shown potential for genetic toxicity and fetal malformation. EGDMA, a component of the excipient acrylates copolymer, was positive for induction of structural chromosomal aberrations in the in vitro chromosomal aberration assay in mammalian cells in the absence of metabolic activation, and negative for genetic toxicity in the Ames assay, and the in vivo mouse micronucleus assay.
In oral fertility studies in rats with tretinoin, the no-observable effect level was 2 mg/kg/day (19 times the MRHD based on BSA comparison).
-
14 CLINICAL STUDIES
14.1 Retin-A Micro (tretinoin) Gel microsphere, 0.1%
In two vehicle-controlled trials, Retin-A Micro (tretinoin) Gel microsphere, 0.1%, applied once daily was significantly more effective than vehicle in reducing the acne lesion counts. The mean reductions in lesion counts from baseline after treatment for 12 weeks are shown in the following table:
Table 1: Mean Percent Reduction in Lesion Counts Retin-A Micro (tretinoin) Gel microsphere, 0.1% Retin-A Micro
(tretinoin) Gel
microsphere, 0.1%Vehicle Gel Study #1
72 ptsStudy #2
71 ptsStudy #1
72 ptsStudy #2
67 ptsNon-inflammatory lesion counts
49%
32%
22%
3%
Inflammatory lesion counts
37%
29%
18%
24%
Total lesion counts
45%
32%
23%
16%
Retin-A Micro (tretinoin) Gel microsphere, 0.1%, was also significantly superior to the vehicle in the investigator's global evaluation of the clinical response. In Study #1, thirty-five percent (35%) of subjects using Retin-A Micro (tretinoin) Gel microsphere, 0.1%, achieved an excellent result, as compared to eleven percent (11%) of subjects on the vehicle control. In Study #2, twenty-eight percent (28%) of patients using Retin-A Micro (tretinoin) Gel microsphere, 0.1%, achieved an excellent result, as compared to nine percent (9%) of the subjects on the vehicle control.
14.2 Retin-A Micro (tretinoin) Gel microsphere, 0.04%
In two vehicle-controlled clinical trials, Retin-A Micro (tretinoin) Gel microsphere, 0.04%, applied once daily, was more effective (p < 0.05) than vehicle in reducing the acne lesion counts. The mean reductions in lesion counts from baseline after treatment for 12 weeks are shown in the following table:
Table 2: Mean Percent Reduction in Lesion Counts Retin-A Micro (tretinoin) Gel microsphere, 0.04% Retin-A Micro (tretinoin)
Gel microsphere, 0.04%Vehicle Gel Study #3
108 ptsStudy #4
111 ptsStudy #3
110 ptsStudy #4
103 ptsNon-inflammatory lesion counts
37%
29%
-2%*
14%
Inflammatory lesion
counts44%
41%
13%
30%
Total lesion counts
40%
35%
8%
20%
* - That is, a mean percent increase of 2%
Retin-A Micro (tretinoin) Gel microsphere, 0.04%, was also superior (p<0.05) to the vehicle in the investigator's global evaluation of the clinical response. In Study #3, fourteen percent (14%) of subjects using Retin-A Micro (tretinoin) Gel microsphere, 0.04%, achieved an excellent result compared to five percent (5%) of subjects on vehicle control. In Study #4, nineteen percent (19%) of subjects using Retin-A Micro (tretinoin) Gel microsphere, 0.04%, achieved an excellent result compared to nine percent (9%) of subjects on vehicle control.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
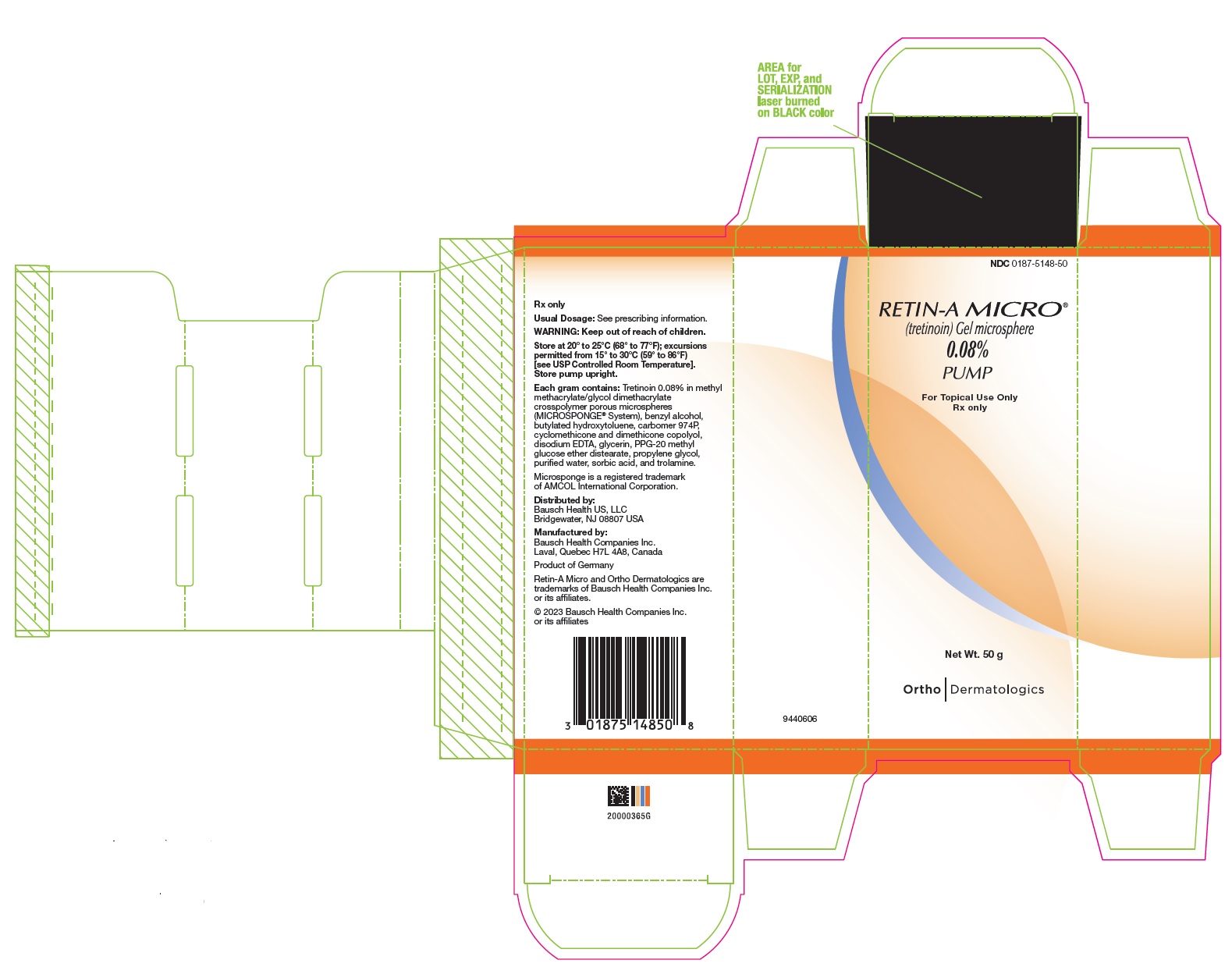
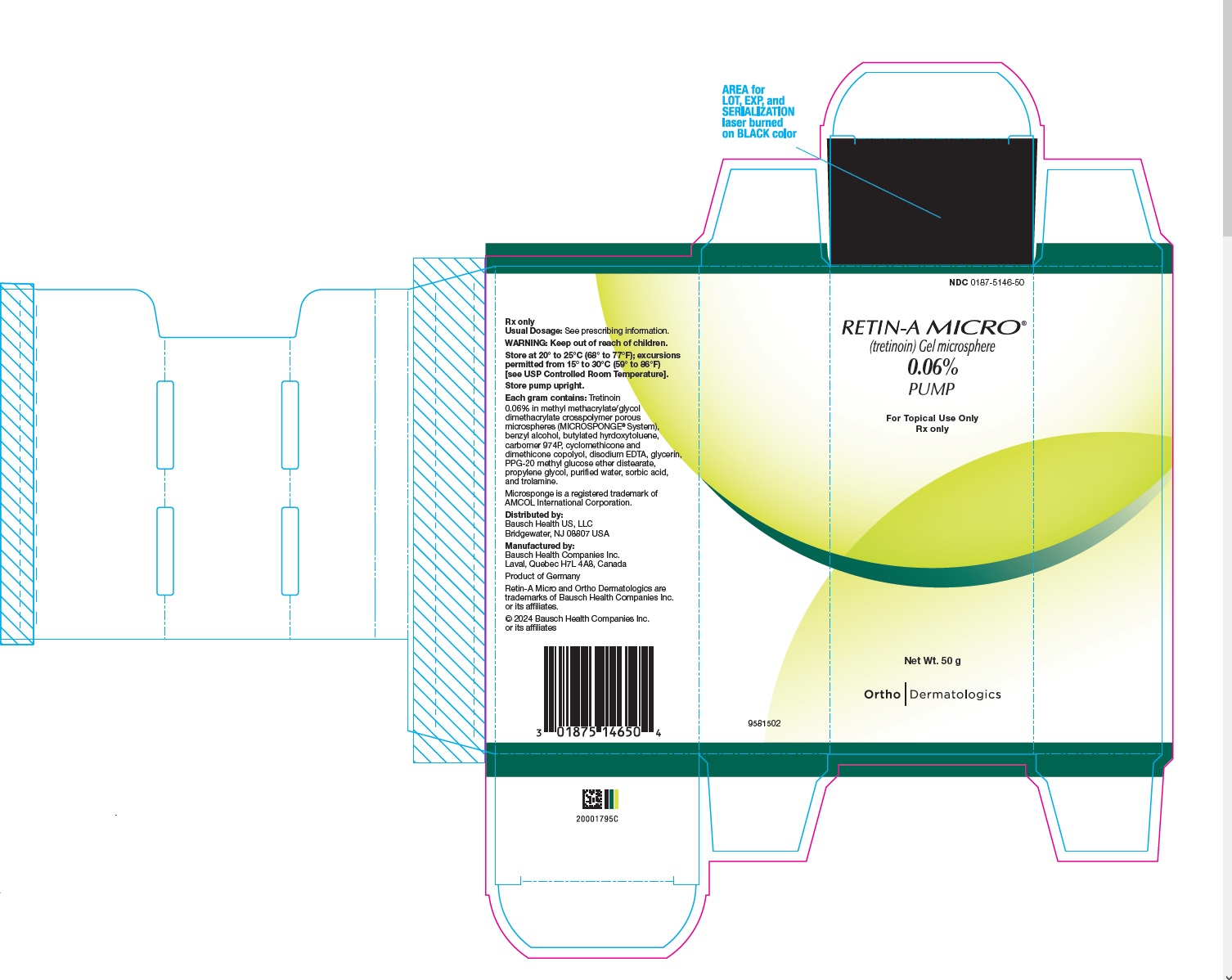
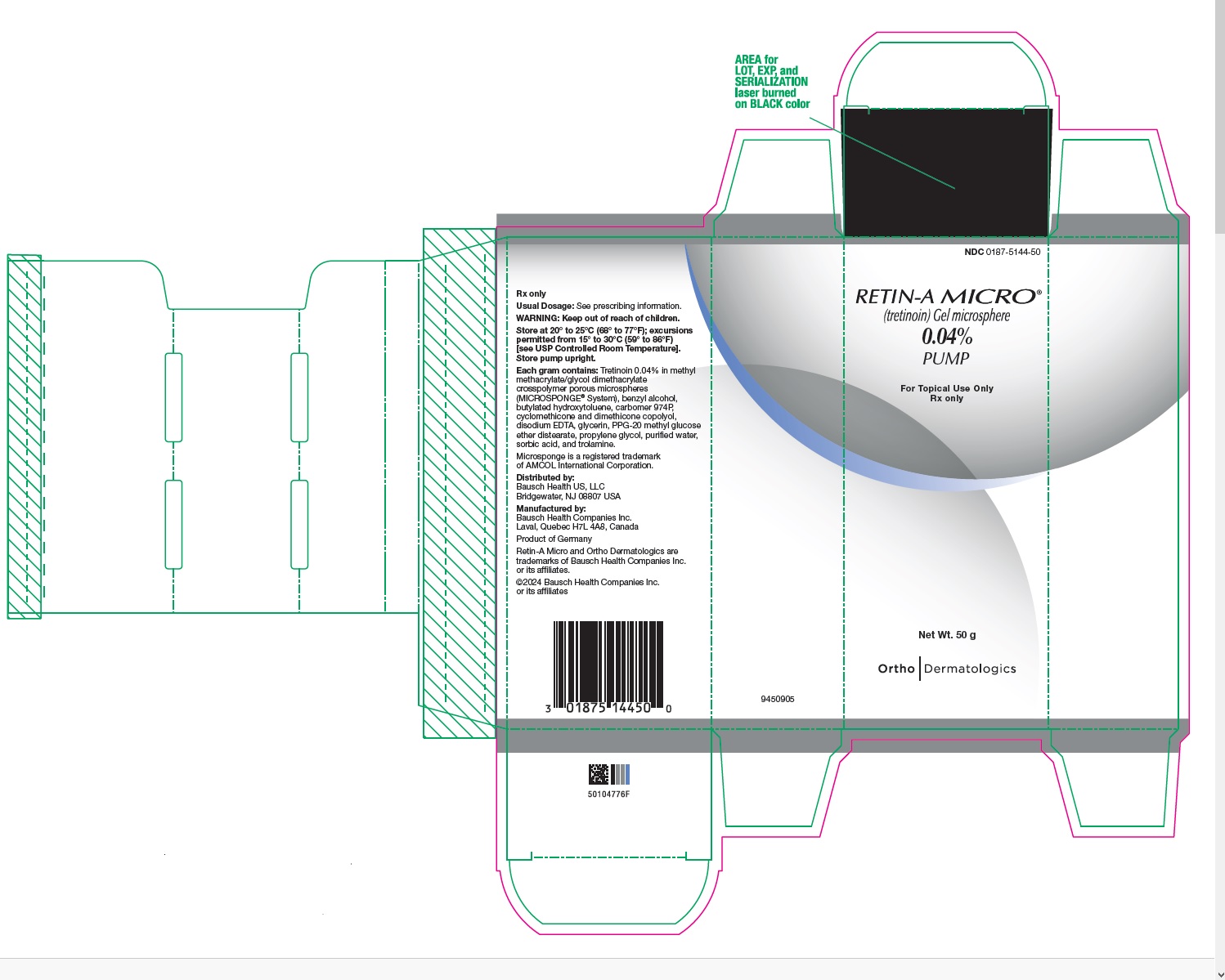
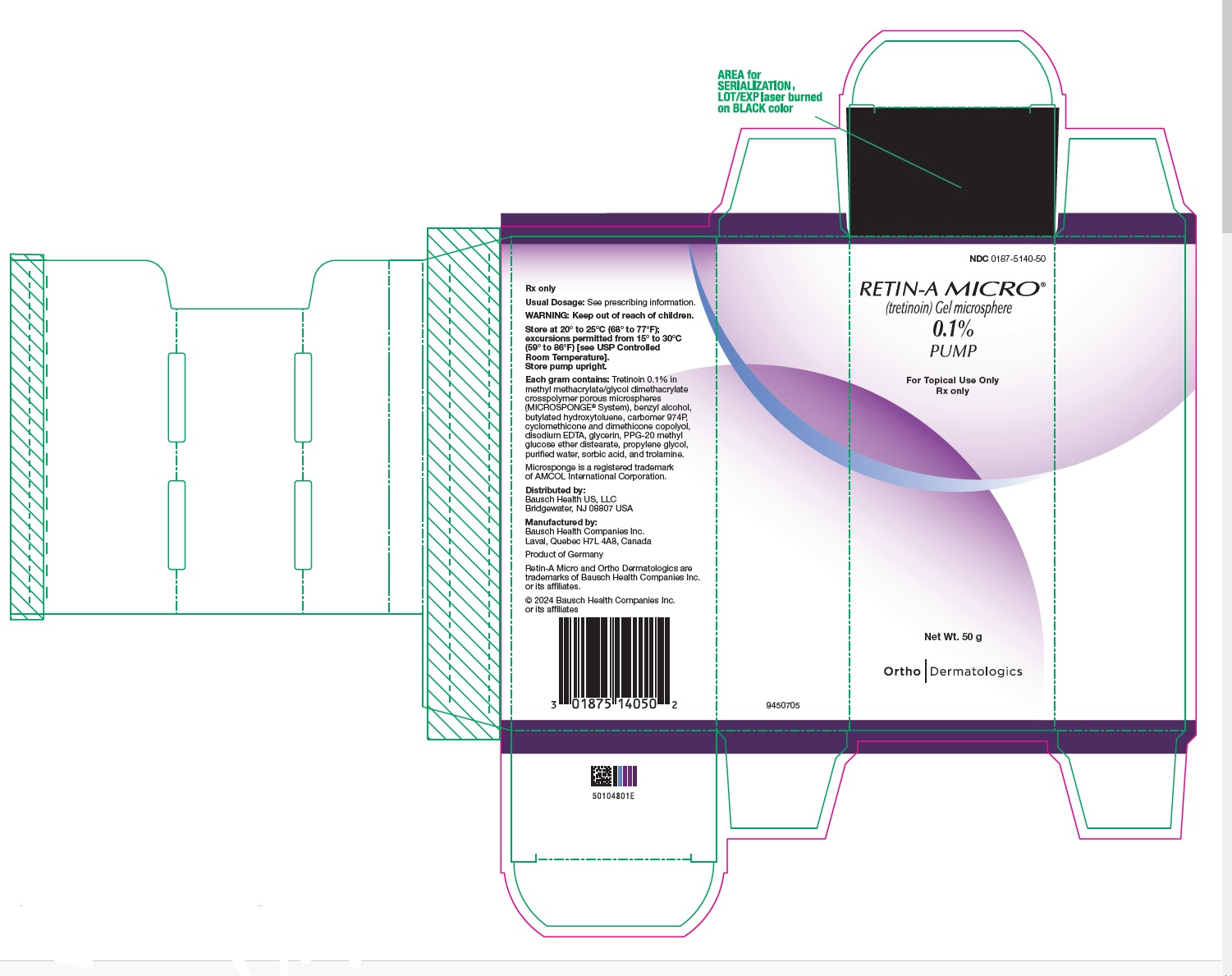
Retin-A Micro Gel is opaque and white to very pale yellow in color.
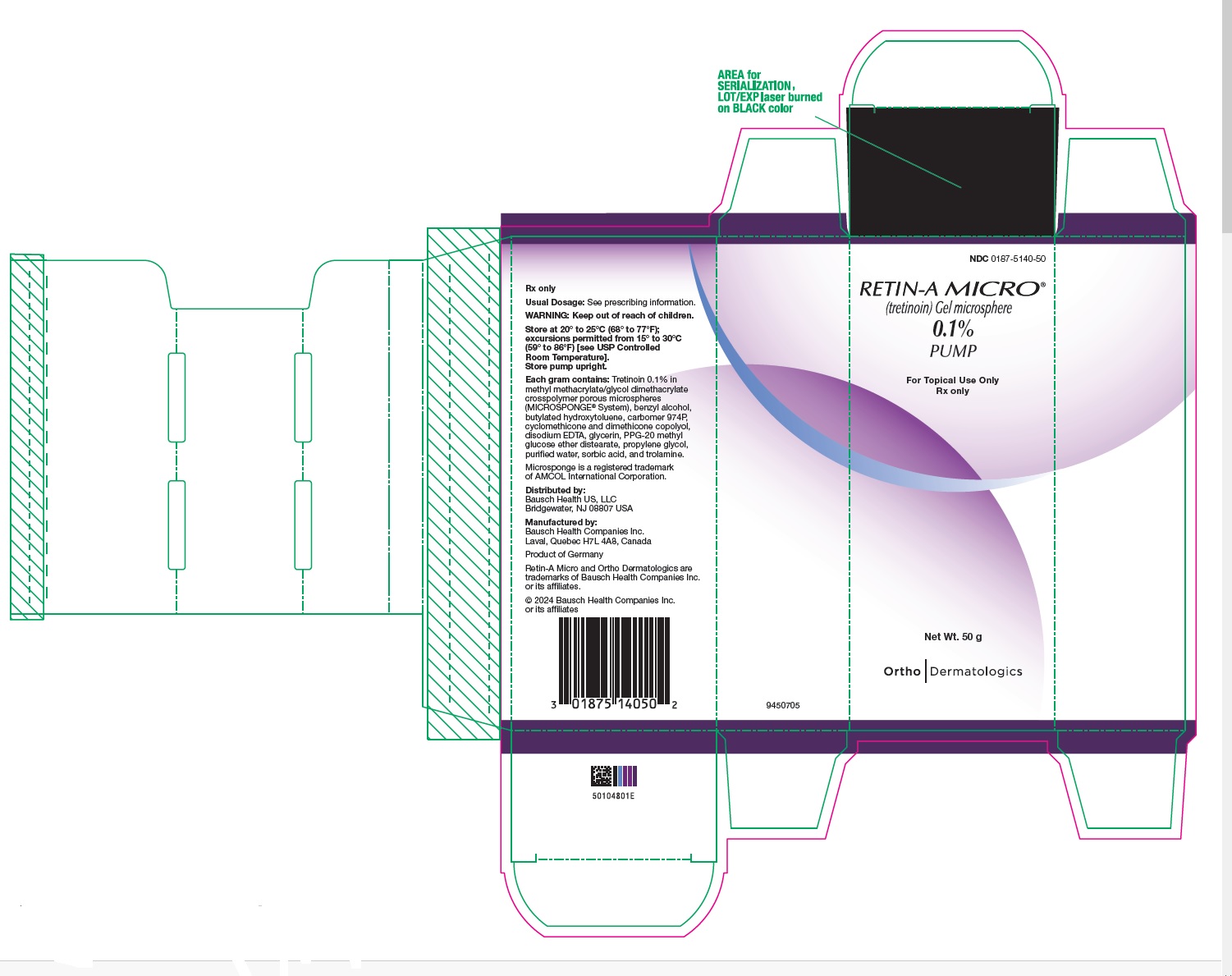
Retin-A Micro Gel, 0.1%, is supplied in
20 gram tube (NDC 0187-5140-20),
45 gram tube (NDC 0187-5140-45) and
50 gram pump (NDC 0187-5140-50).Retin-A Micro Gel, 0.08%, is supplied in
50 gram pump (NDC 0187-5148-50).Retin-A Micro Gel, 0.06%, is supplied in
50 gram pump (NDC 0187-5146-50).Retin-A Micro Gel, 0.04%, is supplied in
20 gram tube (NDC 0187-5144-20),
45 gram tube (NDC 0187-5144-45) and
50 gram pump (NDC 0187-5144-50). -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
The patient should be instructed to:
Cleanse the treatment area thoroughly, before treatment, with a mild, non-medicated cleanser. Do not use more than the recommended amount and do not apply Retin-A Micro more than once daily as this will not produce faster or better results, but may increase irritation.
Minimize exposure to sunlight, including sunlamps. Recommend the use of sunscreen products and protective apparel (e.g., hat) when exposure cannot be avoided.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USAManufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, CanadaRetin-A Micro is a trademark of Bausch Health Companies Inc. or its affiliates.
Microsponge is a registered trademark of AMCOL International Corporation.
All other product/brand names and/or logos are trademarks of the respective owners.© 2024 Bausch Health Companies Inc. or its affiliates
9454706
-
PATIENT INFORMATION
Retin-A Micro ®(ret-in-A MY-kroe)
(tretinoin) Gel microsphere, 0.1%, 0.08%, 0.06%, and 0.04%
for topical useImportant information:Retin-A Micro is for use on skin only. Do not get Retin-A Micro in your eyes, mouth, vagina or the corners of your nose.
What is Retin-A Micro?
Retin-A Microis a prescription medicine used on the skin (topical) to treat acne. Acne is a condition in which the skin has blackheads, whiteheads, and other pimples.
It is not known if Retin-A Micro is safe and effective in the treatment of other conditions.
It is not known if Retin-A Micro is safe and effective in children under 12 years of age.
What should I tell my doctor before using Retin-A Micro?
Before using Retin-A Micro, tell your doctor about all of your medical conditions, including if you:
- have a skin condition called eczema.
- have a sunburn. You should not use Retin-A Micro until your skin has healed.
- have any other medical condition.
- are pregnant or plan to become pregnant. It is not known if Retin-A Micro will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Retin-A Micro passes into your breast milk.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements, and any skin products that you use.
Especially tell your doctor if you use any other medicines to treat your acne, including medicated cleansers or soaps. Using other topical acne products may increase the irritation of your skin when used with Retin-A Micro.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use Retin-A Micro?
- Use Retin-A Micro exactly as your doctor tells you to use it.
- Your doctor may change your dose of Retin-A Micro if you have skin irritation.
- Before you apply Retin-A Micro, gently wash the affected skin area with a mild, non-medicated soap. Rinse and pat your skin dry.
- Apply Retin-A Micro 1 time a day in the evening, or as prescribed by your doctor.
- Do not use more Retin-A Micro than you need to cover the affected area and do not apply Retin-A Micro more than 1 time a day. Using too much Retin-A Micro or using it too often will not give you faster or better results, and you may get skin redness, peeling, or discomfort.
- You may have a brief feeling of warmth or slight stinging after applying Retin-A Micro.
- You may use moisturizers and cosmetics.
- Early in your treatment, you may get new pimples. At this stage, it is important to continue using Retin-A Micro.
- Your acne may not get better right away. Use Retin-A Micro even after your acne improves. Your acne may get better after two weeks of treatment, but more than seven weeks of Retin-A Micro treatment are needed before you get the full benefit.
Applying Retin-A Micro:
- Retin-A Micro comes in a tube and a pump. If you have been prescribed the:
Tube:Squeeze the gel from the tube onto a fingertip. Apply a thin layer to cover the affected area, as prescribed by your doctor. Spread Retin-A Micro evenly over the affected area.
Pump:Fully depress the pump twice to dispense Retin-A Micro onto a fingertip. Apply a thin layer to cover the affected area, as prescribed by your doctor. Spread Retin-A Micro evenly over the affected area.
- Wash your hands after applying Retin-A Micro.
What should I avoid while using Retin-A Micro?
- Avoid washing your skin too often and scrubbing the affected skin area.
- You should avoid sunlamps, tanning beds, and ultraviolet light during treatment with Retin-A Micro.
- Minimize exposure to sunlight.
- If you have to be in the sunlight or are sensitive to sunlight, use a sunscreen with SPF (sun protection factor) of 15 or more and wear a wide-brimmed hat or other protective clothing to cover the treated areas.
- If you do get sunburned, stop using Retin-A Micro until your skin has healed and is back to normal.
- Cold weather and wind may irritate skin treated with Retin-A Micro. Talk to your doctor about ways to manage skin irritation.
- Avoid contact with the peels of limes.
What are the possible side effects of Retin-A Micro?
Retin-A Micro may cause serious side effects, including:
Skin irritation.Retin-A Micro may cause skin dryness, redness, swelling, and blistering. If you develop these symptoms, your doctor may tell you to stop using Retin-A Micro for a while, change your dose, decrease the number of times you apply Retin-A Micro, or completely stop treatment with Retin-A Micro. It is not known if Retin-A Micro is effective when used less than 1 time a day.
The most common side effects of Retin-A Micro includeskin burning and itching.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Retin-A Micro. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Retin-A Micro?
- Store Retin-A Micro at room temperature between 68° to 77°F (20° to 25°C).
- Store Retin-A Micro pump upright.
Keep Retin-A Micro and all medicines out of the reach of children.
General information about the safe and effective use of Retin-A Micro.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Retin-A Micro for a condition for which it was not prescribed. Do not give Retin-A Micro to other people, even if they have the same symptoms you have. It may harm them.
If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Retin-A Micro that is written for health professionals.
For more information, call 1-800-321-4576.
What are the ingredients of Retin-A Micro?
Active ingredient:tretinoin
Inactive ingredients:benzyl alcohol, butylated hydroxytoluene, carbomer 974P, cyclomethicone and dimethicone copolyol, disodium EDTA, glycerin, PPG-20 methyl glucose ether distearate, propylene glycol, purified water, sorbic acid, trolamine, methyl methacrylate/glycol dimethacrylate crosspolymer and propylene glycol dicaprylate/dicaprate
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USAManufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, CanadaRetin-A Micro is a trademark of Bausch Health Companies Inc. or its affiliates.
Microsponge is a registered trademark of AMCOL International Corporation.
All other product/brand names and/or logos are trademarks of the respective owners.© 2024 Bausch Health Companies Inc. or its affiliates
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 09/2024
9454706
- PRINCIPAL DISPLAY PANEL - 0.08% 50 g Carton
- PRINCIPAL DISPLAY PANEL - 0.06% 50 g Carton
- PRINCIPAL DISPLAY PANEL - 0.04% 50 g Carton
- PRINCIPAL DISPLAY PANEL - 0.1% 50 g Carton
-
INGREDIENTS AND APPEARANCE
RETIN-A MICRO
tretinoin gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0187-5140 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 1 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBIC ACID (UNII: X045WJ989B) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0187-5140-20 1 in 1 CARTON 02/07/1997 1 20 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0187-5140-45 1 in 1 CARTON 02/07/1997 2 45 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:0187-5140-50 1 in 1 CARTON 02/07/1997 3 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020475 02/07/1997 RETIN-A MICRO
tretinoin gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0187-5144 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 0.4 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBIC ACID (UNII: X045WJ989B) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0187-5144-20 1 in 1 CARTON 05/10/2002 1 20 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0187-5144-45 1 in 1 CARTON 05/10/2002 2 45 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:0187-5144-50 1 in 1 CARTON 05/10/2002 3 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020475 05/10/2002 RETIN-A MICRO
tretinoin gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0187-5146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 0.6 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) PPG-20 METHYL GLUCOSE ETHER DISTEARATE (UNII: 0057334FAB) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBIC ACID (UNII: X045WJ989B) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0187-5146-50 1 in 1 CARTON 10/23/2017 1 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:0187-5146-02 24 in 1 TRAY 10/23/2017 2 2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020475 10/23/2017 RETIN-A MICRO
tretinoin gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0187-5148 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 0.8 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) CYCLOMETHICONE (UNII: NMQ347994Z) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) PPG-20 METHYL GLUCOSE ETHER DISTEARATE (UNII: 0057334FAB) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBIC ACID (UNII: X045WJ989B) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0187-5148-02 24 in 1 CARTON 01/28/2014 1 2 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0187-5148-50 1 in 1 CARTON 01/28/2014 2 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020475 01/28/2014 Labeler - Bausch Health US LLC (831922468) Establishment Name Address ID/FEI Business Operations Bausch Health Companies Inc. 245141858 manufacture(0187-5140, 0187-5144, 0187-5146, 0187-5148)